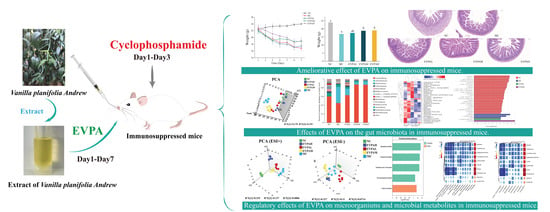
Microbiome–Metabolomic Analysis Revealed the Immunoprotective Effects of the Extract of Vanilla planifolia Andrew (EVPA) on Immunosuppressed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of EVPA
2.2. Animal Experiments
2.3. Organ Index
2.4. Histopathological Observation
2.5. High-Throughput Sequencing Analysis of 16S rDNA
2.6. UPLC–Q-TOF-MS Analysis of Mice Feces
2.7. Statistical Analysis
3. Results
3.1. EVPA Improved the Body Weight of Immunosuppressed Mice
3.2. EVPA Improved the Spleen Index in Immunosuppressed Mice
3.3. Effects of EVPA on Histological Changes in the Small Intestine
3.4. Effect of EVPA on the Intestinal Flora of the Immunosuppressed Mice
3.5. Effects of EVPA on Metabolite Composition in Immunosuppressed Mice
3.6. Effect of EVPA on the Metabolic Pathways of the Intestinal Microbiota
3.7. The correlation between Intestinal Microbiota and Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caruso, R.; Lo, B.C.; Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020, 20, 411–426. [Google Scholar] [CrossRef]
- Da Silva-Maia, J.K.; Batista, Â.G.; Cazarin, C.B.B.; Soares, E.S.; Bogusz Junior, S.; Leal, R.F.; da Cruz-Höfling, M.A.; Maróstica Junior, M.R. Aqueous Extract of Brazilian Berry (Myrciaria jaboticaba) Peel Improves Inflammatory Parameters and Modulates Lactobacillus and Bifidobacterium in Rats with Induced-Colitis. Nutrients 2019, 11, 2776. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut–brain communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef]
- Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The Similarities between Human Mitochondria and Bacteria in the Context of Structure, Genome, and Base Excision Repair System. Molecules 2020, 25, 2857. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Visekruna, A.; Luu, M. The Role of Short-Chain Fatty Acids and Bile Acids in Intestinal and Liver Function, Inflammation, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 703218. [Google Scholar] [CrossRef]
- Di Bella, J.M.; Bao, Y.; Gloor, G.B.; Burton, J.P.; Reid, G. High throughput sequencing methods and analysis for microbiome research. J. Microbiol. Methods 2013, 95, 401–414. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2020, 12, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Van Treuren, W.; Fischer, C.R.; Merrill, B.D.; DeFelice, B.C.; Sanchez, J.M.; Higginbottom, S.K.; Guthrie, L.; Fall, L.A.; Dodd, D.; et al. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature 2021, 595, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, S.; Kaufmann, S.H.E. Infection, inflammation, and chronic diseases: Consequences of a modern lifestyle. Trends Immunol. 2010, 31, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Paudel, K.R.; Sharma, B.; Karki, R. Phytochemical profile and pharmacological activity of Aegle marmelos Linn. J. Integr. Med. 2018, 16, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory Activities of Selected Essential Oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- Grazul, M.; Kwiatkowski, P.; Hartman, K.; Kilanowicz, A.; Sienkiewicz, M. How to Naturally Support the Immune System in Inflammation—Essential Oils as Immune Boosters. Biomedicines 2023, 11, 2381. [Google Scholar] [CrossRef]
- Khoyratty, S.; Kodja, H.; Verpoorte, R. Vanilla flavor production methods: A review. Ind. Crops Prod. 2018, 125, 433–442. [Google Scholar] [CrossRef]
- Takahashi, M.; Inai, Y.; Miyazawa, N.; Kurobayashi, Y.; Fujita, A. Identification of the Key Odorants in Tahitian Cured Vanilla Beans (Vanilla tahitensis) by GC-MS and an Aroma Extract Dilution Analysis. Biosci. Biotechnol. Biochem. 2013, 77, 601–605. [Google Scholar] [CrossRef]
- Bilcu, M.; Grumezescu, A.; Oprea, A.; Popescu, R.; Mogoșanu, G.; Hristu, R.; Stanciu, G.; Mihailescu, D.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of Vanilla, Patchouli and Ylang Ylang Essential Oils Stabilized by Iron Oxide@C14 Nanostructures against Bacterial Adherence and Biofilms Formed by Staphylococcus aureus and Klebsiella pneumoniae Clinical Strains. Molecules 2014, 19, 17943–17956. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.; Liu, H.; Liu, H.; Xiang, S.; You, F.; Jiang, Y.; Lin, J.; Zhang, D.; Zheng, C. Pharmacologic effects approach of essential oils and their components on respiratory diseases. J. Ethnopharmacol. 2023, 304, 115962. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, X.; Zhan, J.; You, Y.; Huang, W. Vanillin Alleviates High Fat Diet-Induced Obesity and Improves the Gut Microbiota Composition. Front. Microbiol. 2018, 9, 2733. [Google Scholar] [CrossRef]
- Yadav, M.; Pandey, R.; Chauhan, N.S. Catabolic Machinery of the Human Gut Microbes Bestow Resilience Against Vanillin Antimicrobial Nature. Front. Microbiol. 2020, 11, 588545. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Kosyan, D.B.; Inchagova, K.S.; Duskaev, G.K. Plant-Derived Quorum Sensing Inhibitors (Quercetin, Vanillin and Umbelliferon) Modulate Cecal Microbiome, Reduces Inflammation and Affect Production Efficiency in Broiler Chickens. Microorganisms 2023, 11, 1326. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhu, L.; Wang, P.; Xu, F.; Zhang, Y. Octenyl Succinic Acid Starch-Stabilized Vanilla Essential Oil Pickering Emulsion: Preparation, Characterization, Antioxidant Activity, and Storage Stability. Foods 2022, 11, 987. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, K.; Zeng, S.; Zheng, Y.; Cao, J.; Li, C. Microbiome-Metabolomics Analysis Reveals the Mechanism of Holothuria leucospilota Polysaccharides (HLP) in Ulcerative Colitis. Mol. Nutr. Food Res. 2023, 67, e2200633. [Google Scholar] [CrossRef]
- Yu, G.; Xu, C.; Zhang, D.; Ju, F.; Ni, Y. MetOrigin: Discriminating the origins of microbial metabolites for integrative analysis of the gut microbiome and metabolome. IMeta 2022, 1, e10. [Google Scholar] [CrossRef]
- Abd El-Aziz, R.; Naguib, M.; Rashed, L.A. Spleen size in patients with metabolic syndrome and its relation to metabolic and inflammatory parameters. Egypt. J. Intern. Med. 2018, 30, 78–82. [Google Scholar] [CrossRef]
- Ji, S.Y.; Lee, H.; Hwangbo, H.; Kim, M.Y.; Kim, D.H.; Park, B.S.; Koo, Y.T.; Kim, J.S.; Lee, K.W.; Ko, J.C.; et al. Agarwood Pill Enhances Immune Function in Cyclophosphamide-induced Immunosuppressed Mice. Biotechnol. Bioprocess Eng. 2023, 28, 63–73. [Google Scholar] [CrossRef]
- Gurcan, M.N.; Boucheron, L.E.; Can, A.; Madabhushi, A.; Rajpoot, N.M.; Yener, B. Histopathological Image Analysis: A Review. IEEE Rev. Biomed. Eng. 2009, 2, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.; Zhao, Q.; Wen, A.; Li, L.; Zhang, Y. The regulating effect of Tibet Opuntia ficus-indica (Linn.) Mill. polysaccharides on the intestinal flora of cyclophosphamide-induced immunocompromised mice. Int. J. Biol. Macromol. 2022, 207, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, H.; Chen, Y.; Ma, C.; Zhang, Q.; Li, H.; Xie, Z.; Hong, Y. Targeting the gut microbiota to alleviate chemotherapy-induced toxicity in cancer. Crit. Rev. Microbiol. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ma, T.; Zhang, X.; Zhao, Q.; Zhu, K.; Cao, J.; Liu, Z.; Shen, X.; Li, C. Holothuria leucospilota Polysaccharides Improve Immunity and the Gut Microbiota in Cyclophosphamide-Treated Immunosuppressed Mice. Mol. Nutr. Food Res. 2022, 67, e2200317. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Banu, H.H.; Ramya Bai, R.M.; Shanmugavalli, R. Biochemical evaluation of antihyperglycemic and antioxidant nature of Psidium guajava leaves extract in streptozotocin-induced experimental diabetes in rats. Pharm. Biol. 2009, 47, 298–303. [Google Scholar] [CrossRef]
- Delfani, S.; Mohammadrezaei Khorramabadi, R.; Ghamari, S.; Boroujeni, R.K.; Khodabandeloo, N.; Khorzoughi, M.G.; Shahsavari, S. Systematic review for phytotherapy in Streptococcus mutans. J. Pharm. Sci. Res. 2017, 9, 552–561. [Google Scholar]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Raichon, L.; Venzon, M.; Klein, J.; Axelrad, J.E.; Zhang, C.; Sullivan, A.P.; Hussey, G.A.; Casanovas-Massana, A.; Noval, M.G.; Valero-Jimenez, A.M.; et al. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat. Commun. 2022, 13, 5926. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Li, R.; Tao, T.; Zhong, R.; Du, H.; Liao, Z.; Sun, Z.; Xu, C. Therapeutic potential of Litsea cubeba essential oil in modulating inflammation and the gut microbiome. Front. Microbiol. 2023, 14, 1233934. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, B.; Li, W.; Geng, F.; Gao, X.; Yue, L.; Liu, H.; Liu, C.; Su, Z.; Lü, J.; et al. Protopanaxadiol manipulates gut microbiota to promote bone marrow hematopoiesis and enhance immunity in cyclophosphamide-induced immunosuppression mice. MedComm 2023, 4, e222. [Google Scholar] [CrossRef] [PubMed]
- van der Haar Àvila, I.; Windhouwer, B.; van Vliet, S.J. Current state-of-the-art on ganglioside-mediated immune modulation in the tumor microenvironment. Cancer Metastasis Rev. 2023, 42, 941–958. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luu, L.D.W.; Wang, X.; Cui, X.; Huang, X.; Fu, J.; Zhu, X.; Li, Z.; Wang, Y.; Tai, J. Metabolomic analysis reveals potential biomarkers and the underlying pathogenesis involved in Mycoplasma pneumoniae pneumonia. Emerg. Microbes Infect. 2022, 11, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, W.-W.; Wu, R.-T.; Song, Y.-H.; Wu, W.-Y.; Yin, S.-H.; Li, W.-J.; Xie, M.-Y. Protective effect of Ganoderma atrum polysaccharides in acute lung injury rats and its metabolomics. Int. J. Biol. Macromol. 2020, 142, 693–704. [Google Scholar] [CrossRef]
- Czumaj, A.; Szrok-Jurga, S.; Hebanowska, A.; Turyn, J.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The Pathophysiological Role of CoA. Int. J. Mol. Sci. 2020, 21, 9057. [Google Scholar] [CrossRef]
- Tóth, F.; Cseh, E.K.; Vécsei, L. Natural Molecules and Neuroprotection: Kynurenic Acid, Pantethine and α-Lipoic Acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Besson, S.; Almeida, M.G.; Silveira, C.M. Nitrite reduction in bacteria: A comprehensive view of nitrite reductases. Coord. Chem. Rev. 2022, 464, 214560. [Google Scholar] [CrossRef]







| Number | Compound | Molecular Formula | Relative Content (%) |
|---|---|---|---|
| 1 | Styrene | C8H8 | 0.23 ± 0.14 |
| 2 | 2,2,4,6,6-pentamethyl-heptane | C12H26 | 0.15 ± 0.11 |
| 3 | 4-methoxy-benzaldehyde | C8H8O2 | 0.14 ± 0.10 |
| 4 | 4-methoxy-benzenemethanol | C8H10O2 | 8.69 ± 2.13 |
| 5 | 4-methoxy-benzoicacidmethylester | C9H10O3 | 0.17 ± 0.12 |
| 6 | 3-phenyl-2-Propenoicacidmethylester(E)- | C10H10O2 | 0.03 ± 0.01 |
| 7 | Vanillin | C8H8O3 | 30.54 ± 1.59 |
| 8 | Caryophyllene | C15H24 | 0.10 ± 0.08 |
| 9 | 4-methoxy-benzenemethanolacetate | C10H12O3 | 0.18 ± 0.11 |
| 10 | 4-methoxybenzoicacid | C8H8O3 | 0.84 ± 0.08 |
| 11 | Dodecanoic acid | C12H24O2 | 0.06 ± 0.01 |
| 12 | Myristic acid | C14H28O2 | 0.08 ± 0.05 |
| 13 | 2-Pentadecanone,6,10,14-trimethyl- | C18H36O | 0.12 ± 0.06d |
| 14 | Hexadecanoic acid, methyl ester | C17H34O2 | 0.12 ± 0.07d |
| 15 | n-Hexadecanoic acid | C16H32O2 | 4.83 ± 0.12 |
| 16 | Hexadecanoicacid, ethylester | C18H36O2 | 0.15 ± 0.07 |
| 17 | 9,12-Octadecadienoicacid(Z,Z)-,methyl ester | C19H34O2 | 0.65 ± 0.26 |
| 18 | 11-Octadecenoicacid, methyl ester | C19H36O2 | 0.17 ± 0.11 |
| 19 | Linoleic acid | C18H32O2 | 3.81 ± 2.59 |
| 20 | 9,12-Octadecadienoicacid, ethyl ester | C20H36O2 | 1.16 ± 0.58 |
| 21 | Oleic Acid | C18H34O2 | 0.73 ± 0.45 |
| 22 | Heneicosane | C21H44 | 0.56 ± 0.06 |
| 23 | Tetracosane | C24H50 | 1.39 ± 0.98 |
| 24 | Nonadecane-2,4-dione | C19H36O2 | 1.06 ± 0.77 |
| 25 | 1-Octacosanol | C28H58O | 1.58 ± 0.56 |
| 26 | Octacosane | C28H58 | 4.31 ± 0.35 |
| 27 | Heptacosane | C27H56 | 3.43 ± 0.44 |
| Model | Group Comparison | R2 Y (cum) | Q2 (cum) |
|---|---|---|---|
| ESI+ | MC VS NC | 0.999 | 0.972 |
| ESI+ | MC VS EVPAL | 0.996 | 0.880 |
| ESI+ | MC VS EVPAM | 0.998 | 0.960 |
| ESI+ | MC VS EVPAH | 1 | 0.962 |
| ESI− | MC VS NC | 0.999 | 0.956 |
| ESI− | MC VS EVPAL | 0.993 | 0.808 |
| ESI− | MC VS EVPAM | 0.998 | 0.776 |
| ESI− | MC VS EVPAH | 0.997 | 0.851 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Y.; Zhu, K.; Li, C.; Zhao, Q.; Gu, F.; Xu, F.; Chu, Z. Microbiome–Metabolomic Analysis Revealed the Immunoprotective Effects of the Extract of Vanilla planifolia Andrew (EVPA) on Immunosuppressed Mice. Foods 2024, 13, 701. https://doi.org/10.3390/foods13050701
Zhang X, Li Y, Zhu K, Li C, Zhao Q, Gu F, Xu F, Chu Z. Microbiome–Metabolomic Analysis Revealed the Immunoprotective Effects of the Extract of Vanilla planifolia Andrew (EVPA) on Immunosuppressed Mice. Foods. 2024; 13(5):701. https://doi.org/10.3390/foods13050701
Chicago/Turabian StyleZhang, Xin, Yunlong Li, Kexue Zhu, Chuan Li, Qingyun Zhao, Fenglin Gu, Fei Xu, and Zhong Chu. 2024. "Microbiome–Metabolomic Analysis Revealed the Immunoprotective Effects of the Extract of Vanilla planifolia Andrew (EVPA) on Immunosuppressed Mice" Foods 13, no. 5: 701. https://doi.org/10.3390/foods13050701