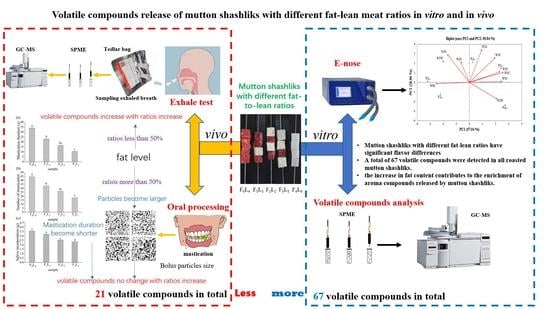
Effect of Fat to Lean Meat Ratios on the Formation of Volatile Compounds in Mutton Shashliks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mutton Shashlik Preparation
2.2. E-Nose Analysis
2.3. Volatile Compounds
2.3.1. HS-SPME Analysis of Mutton Shashliks before Consumption
2.3.2. Identification of Volatile Compounds
2.3.3. Subjects
2.3.4. Oral Processing Parameters
2.3.5. Bolus Collection and Saliva Incorporation
2.3.6. Sieve Analysis of Meat Bolus
2.3.7. Image Analysis of Meat Particles
2.4. Exhaled Breath Collection and Analysis
2.5. Statistical Analyses
3. Results and Discussion
3.1. Moisture, Protein, and Fat Content
3.2. E-Nose
3.3. Volatile Compounds of Mutton Shashliks with Different Fat–Lean Ratios
3.4. Oral Processing
3.4.1. Mastication Duration and Number
3.4.2. Saliva Incorporation
3.4.3. Sieve Analysis of Meat Bolus
3.5. Exhaled Breath Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Hui, T.; Zheng, X.; Li, S.; Wei, X.; Li, P.; Zhang, D.; Wang, Z. Characterization of key lipids for binding and generating aroma compounds in roasted mutton by UPLC-ESI-MS/MS and Orbitrap Exploris GC. Food Chem. 2022, 374, 131723. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Al-Dalali, S.; Wang, J.; Xie, J.; Shakoor, A.; Asimi, S.; Shah, H.; Patil, P. Aroma compounds identified in cooked meat: A review. Food Res. Int. 2022, 157, 111385. [Google Scholar] [CrossRef] [PubMed]
- Ortner, E.; Granvogl, M. Thermally Induced Generation of Desirable Aroma-Active Compounds from the Glucosinolate Sinigrin. J. Agric. Food Chem. 2018, 66, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik-Kalinowska, I.; Szpicer, A.; Binkowska, W.; Hanula, M.; Marcinkowska-Lesiak, M.; Poltorak, A. Effect of Processing on Volatile Organic Compounds Formation of Meat—Review. Appl. Sci. 2023, 13, 705. [Google Scholar] [CrossRef]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of cooking methods on changes in fatty acids contents, lipid oxidation and volatile compounds of rabbit meat. LWT 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T.; Sun, J. Comprehensive Evaluation of Volatile and Nonvolatile Compounds in Oyster Cuts of Roasted Lamb at Different Processing Stages Using Traditional Nang Roasting. Foods 2021, 10, 1508. [Google Scholar] [CrossRef]
- Frank, D.C.; Ball, A.J.; Hughes, J.M.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R.D. Sensory and flavor chemistry characteristics of Australian beef: Influence of intramuscular fat, feed, and breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef]
- Myers, A.J.; Scramlin, S.M.; Dilger, A.C.; Souza, C.M.; McKeith, F.K.; Killefer, J. Contribution of lean, fat, muscle color and degree of doneness to pork and beef species flavor. Meat Sci. 2009, 82, 59–63. [Google Scholar] [CrossRef]
- Sink, J.D.; Caporaso, F. Lamb and mutton flavour: Contributing factors and chemical aspects. Meat Sci. 1977, 1, 119–127. [Google Scholar] [CrossRef]
- Berry, B. Fat level, high temperature cooking and degree of doneness affect sensory, chemical and physical properties of beef patties. J. Food Sci. 1994, 59, 10–14. [Google Scholar] [CrossRef]
- Pu, D.; Shan, Y.; Wang, J.; Sun, B.; Xu, Y.; Zhang, W.; Zhang, Y. Recent trends in aroma release and perception during food oral processing: A review. Crit. Rev. Food Sci. 2022, 1–17. [Google Scholar] [CrossRef]
- Labouré, H.; Repoux, M.; Courcoux, P.; Feron, G.; Guichard, E. Inter-individual retronasal aroma release variability during cheese consumption: Role of food oral processing. Food Res. Int. 2014, 64, 692–700. [Google Scholar] [CrossRef]
- Horwitz, W. Methods 930.15, 942.05, 920.39, 954.01, 991.43, 50.1.14, 9.1.09. In Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Gong, H.; Yang, Z.; Liu, M.; Shi, Z.; Li, J.; Chen, W.; Qiao, X. Time-dependent categorization of volatile aroma compound formation in stewed Chinese spicy beef using electron nose profile coupled with thermal desorption GC–MS detection. Food Sci. Hum. Well. 2017, 6, 137–146. [Google Scholar] [CrossRef]
- Vasta, V.; Ventura, V.; Luciano, G.; Andronico, V.; Pagano, R.I.; Scerra, M.; Biondi, L.; Avondo, M.; Priolo, A. The volatile compounds in lamb fat are affected by the time of grazing. Meat Sci. 2012, 90, 451–456. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Song, H. Variation in Volatile Flavor Compounds of Cooked Mutton Meatballs during Storage. Foods 2021, 10, 2430. [Google Scholar] [CrossRef]
- Pematilleke, N.; Kaur, M.; Adhikari, B.; Torley, P. Influence of meat texture on oral processing and bolus formation. J. Food Eng. 2020, 283, 110038. [Google Scholar] [CrossRef]
- Ling, J.; Xu, J.; Lin, H.; Lin, J. Prediction and fusion algorithm for meat moisture content measurement based on loss-on-drying method. Int. J. Agric. Biol. Eng. 2020, 13, 198–204. [Google Scholar] [CrossRef]
- Kang, N. Comparison of Sampling Methods to Determine Methane in Tedlar Bag Directly Against High Pressurized Calibration Gas Cylinder by Gas Chromatography. SSRN Electron. J. 2023, 4313962. [Google Scholar] [CrossRef]
- Phillips, M.; Gleeson, K.; Hughes, J.M.B.; Greenberg, J.; Cataneo, R.N.; Baker, L.; McVay, W.P. Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 1999, 353, 1930–1933. [Google Scholar] [CrossRef]
- Ross, B.M.; Babgi, R. Volatile compounds in blood headspace and nasal breath. J. Breath Res. 2017, 11, 046001. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Liu, Q.; Xia, X.; Wang, Y.; Kong, B. Effect of different types of smoking materials on the flavor, heterocyclic aromatic amines, and sensory property of smoked chicken drumsticks. Food Chem. 2022, 367, 130680. [Google Scholar] [CrossRef] [PubMed]
- Mi, R.; Chen, X.; Xiong, S.; Qi, B.; Li, J.; Qiao, X.; Chen, W.; Qu, C.; Wang, S. Predominant yeasts in Chinese Dong fermented pork (Nanx Wudl) and their aroma-producing properties in fermented sausage condition. Food Sci. Hum. Well. 2021, 10, 231–240. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Pan, T.; Suleman, R.; Wang, Z.; Zhang, D. Effects of roasting by charcoal, electric, microwave and superheated steam methods on (non) volatile compounds in oyster cuts of roasted lamb. Meat Sci. 2021, 172, 108324. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Ling, L.C.; Teranishi, R.; Mon, T.R. Roasted lamb fat: Basic volatile components. J. Agric. Food Chem. 1977, 25, 1227–1229. [Google Scholar] [CrossRef]
- Liu, H.; Hui, T.; Fang, F.; Li, S.; Wang, Z.; Zhang, D. The formation of key aroma compounds in roasted mutton during the traditional charcoal process. Meat Sci. 2022, 184, 108689. [Google Scholar] [CrossRef]
- Wasserman, A.E.; Talley, F. Organoleptic Identification of Roasted Beef, Veal, Lamb and Pork as Affected by Fat. J. Food Sci. 1968, 33, 219–223. [Google Scholar] [CrossRef]
- Pearson, A.M.; Love, J.D.; Shorland, F.B. “Warmed-Over” Flavor in Meat, Poultry, and Fish. Adv. Food Res. 1977, 23, 1–74. [Google Scholar]
- Elmore, J.S.; Mottram, D.S.; Enser, M.; Wood, J.D. Effect of the polyunsaturated fatty acid composition of beef muscle on the profile of aroma volatiles. J. Agric. Food Chem. 1999, 47, 1619–1625. [Google Scholar] [CrossRef]
- Zou, Y.; Kang, D.; Rui, L.; Qi, J.; Zhang, W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochem. 2018, 46, 36–45. [Google Scholar] [CrossRef]
- Shen, D.; Li, M.; Song, H.; Zou, T.; Zhang, L.; Xiong, J. Characterization of aroma in response surface optimized no-salt bovine bone protein extract by switchable GC/GC×GC-olfactometry-mass spectrometry, electronic nose, and sensory evaluation. LWT 2021, 147, 111559. [Google Scholar] [CrossRef]
- Kerth, C. Determination of volatile aroma compounds in beef using differences in steak thickness and cook surface temperature. Meat Sci. 2016, 117, 27–35. [Google Scholar] [CrossRef]
- Guo, Q. Understanding the oral processing of solid foods: Insights from food structure. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2941–2967. [Google Scholar] [CrossRef]
- Liu, D.; Deng, Y.; Gong, A.; Han, Y.H.; Wang, H. Impact of the Breakdown Behavior on Chinese Traditional Stewed Pork with Brown Sauce: Physical Properties Using Microstructural Analysis. J. Food Qual. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food-saliva interactions: Mechanisms and implications. Trends Food Sci. Technol. 2017, 66, 125–134. [Google Scholar] [CrossRef]
- Frank, D.; Kaczmarska, K.; Paterson, J.; Piyasiri, U.; Warner, R. Effect of marbling on volatile generation, oral breakdown and in mouth flavor release of grilled beef. Meat Sci. 2017, 133, 61–68. [Google Scholar] [CrossRef]
- Peyron, M.A.; Mishellany, A.; Woda, A. Particle size distribution of food boluses after mastication of six natural foods. J. Dent. Res. 2004, 83, 578–582. [Google Scholar] [CrossRef]
- Hoebler, M.-F.; Devaux, A.; Karinthi, C.; Belleville, J.-L.; Barry, C. Particle size of solid food after human mastication and in vitro simulation of oral breakdown. Int. J. Food Sci. Nutr. 2000, 51, 353–366. [Google Scholar] [CrossRef]
- Marine, D.; Derks, J.; Ketel, E.C.; Wijk, R.D.; Stieger, M. Eating behaviour explains differences between individuals in dynamic texture perception of sausages. Food Qual. Prefer. 2015, 41, 189–200. [Google Scholar]
- Mioche, L.; Bourdiol, P.; Monier, S.; Martin, J.-F. The relationship between chewing activity and food bolus properties obtained from different meat textures. Food Qual. Prefer. 2002, 13, 583–588. [Google Scholar] [CrossRef]
- Okawa, J.; Hori, K.; Yoshimoto, T.; Salazar, S.E.; Ono, T. Higher Masticatory Performance and Higher Number of Chewing Strokes Increase Retronasal Aroma. Front. Nutr. 2021, 8, 623507. [Google Scholar] [CrossRef]
- Canon, F.; Neiers, F.; Guichard, E. Saliva and Flavor Perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, R.; Zhao, Y.; Sun, J.; Zhang, Y.; Li, H.; Zhao, D.; Wang, B.; Ye, X.; Sun, B. Characterization of the aroma release from retronasal cavity and flavor perception during baijiu consumption by Vocus-PTR-MS, GC×GC-MS, and TCATA analysis. LWT 2023, 174, 114430. [Google Scholar] [CrossRef]
- Lu, C.; Zhe, W.; Pla, B.; Al, C.; Yz, D.; Hla, B.; Jsa, B. The effect of saliva on the aroma release of esters in simulated baijiu under the impact of high ethanol concentration. J. Food Compos. Anal. 2021, 104, 104134. [Google Scholar]
- Paola, P.; Alessandro, G.; Silvia, E.; Luigi, M.; Paolo, C.P.; Angela, C.; Valeria, S.; Elisabetta, M.; Smith, D.P.; Owens, S.M. Saliva from Obese Individuals Suppresses the Release of Aroma Compounds from Wine. PLoS ONE 2014, 9, e85611. [Google Scholar]




| F0L4 | F1L3 | F2L2 | F3L1 | F4L0 | |
|---|---|---|---|---|---|
| Moisture | 53.28 ± 2.81 a | 42.13 ± 2.31 b | 31.57 ± 2.19 c | 20.75 ± 1.31 d | 9.09 ± 1.24 e |
| Protein | 41.17 ± 1.71 a | 31.34 ± 1.56 b | 21.03 ± 1.97 c | 11.20 ±1.03 d | 1.01 ± 0.27 e |
| Fat | 3.54 ± 0.62 e | 25.26 ± 1.48 d | 46.21 ± 2.53 c | 66.24 ± 3.17 b | 87.81 ±4.28 a |
| Volatile Compounds | LRI * | Identification + | F0L4 | F1L3 | F2L2 | F3L1 | F4L0 | |
|---|---|---|---|---|---|---|---|---|
| Literature | Calculated | |||||||
| Hexanal | 800 | 802 | MS + LRI | 150.47 ± 12.65 d | 649.30 ± 7.76 c | 687.64 ± 16.91 b | 697.77 ± 8.84 b | 713.10 ± 7.36 a |
| Heptanal | 900 | 904 | MS + LRI | 119.55 ± 6.65 e | 261.82 ± 8.97 d | 313.80 ± 7.80 c | 339.42 ± 6.73 b | 380.33 ± 3.79 a |
| 2-hexen-1-al | 854 | 860 | MS + LRI | ND | ND | ND | ND | 111.84 ± 11.77 |
| Benzaldehyde | 959 | 963 | MS + LRI | 98.30 ± 9.89 d | 246.00 ± 12.37 c | 435.32 ± 10.63 b | 443.25 ± 19.60 b | 515.44 ± 11.54 a |
| Octanal | 1004 | 1005 | MS + LRI | 124.99 ± 2.93 e | 476.33 ± 3.74 d | 582.55 ± 8.12 c | 629.81 ± 3.20 b | 642.84 ± 6.07 a |
| (E, E)-2,4-Heptadienal | 1011 | 1012 | MS + LRI | ND | ND | ND | ND | 51.65 ± 9.70 |
| Phenylacetaldehyde | 1051 | 1053 | MS + LRI | ND | ND | 225.08 ± 15.24 c | 331.29 ± 12.58 b | 491.30 ± 12.38 a |
| (E)-2-Octenal | 1065 | 1067 | MS + LRI | ND | 144.96 ± 37.60 d | 291.13 ± 9.23 c | 346.03 ± 11.25 b | 335.25 ± 10.39 a |
| Nonanal | 1112 | 1114 | MS + LRI | 139.87 ± 18.15 d | 517.66 ± 12.06 c | 612.80 ± 17.22 b | 636.29 ± 15.17 a | 646.76 ± 10.22 a |
| (E)-2-Nonenal | 1163 | 1167 | MS + LRI | ND | 88.63 ± 12.87 e | 190.62 ± 10.13 b | 164.51 ± 16.77 c | 276.85 ± 12.91 a |
| Decanal | 1202 | 1205 | MS + LRI | ND | 110.67 ± 21.46 d | 215.67 ± 11.67 c | 269.43 ± 16.76 b | 281.88 ± 10.61 a |
| (E, E)-2,4-Nonadienal | 1212 | 1216 | MS + LRI | ND | 359.43 ± 20.46 | ND | ND | ND |
| Cuminaldehyde | 1238 | 1240 | MS + LRI | ND | 45.54 ± 28.03 d | 55.90 ± 3.61 c | 59.61 ± 4.78 b | 61.22 ± 6.58 a |
| (2E)-2-Decenal | 1265 | 1268 | MS + LRI | ND | 51.56 ± 20.95 c | 179.75 ± 27.12 b | 187.60 ± 14.19 b | 420.70 ± 17.54 a |
| 2,4-Decadienal | 1295 | 1299 | MS + LRI | ND | ND | ND | ND | 28.83 ± 2.51 |
| Undecanal | 1305 | 1307 | MS + LRI | ND | ND | 142.98 ± 14.17 a | 85.58 ± 13.02 b | 72.49 ± 14.18 c |
| Undecenal | 1359 | 1360 | MS + LRI | ND | ND | 64.86 ± 8.36 b | 72.74 ± 8.98 b | 141.57 ± 11.02 a |
| Dodecanal | 1407 | 1410 | MS + LRI | ND | ND | 15.51 ± 7.62 b | 24.00 ± 4.25 b | 28.05 ± 6.94 a |
| Pentadecanal | 1715 | 1718 | MS + LRI | ND | ND | ND | 31.01 ± 11.82 | 34.10 ± 12.51 |
| (E)-4-Nonenal | - | 1435 | MS | ND | ND | ND | ND | 40.84 ± 12.88 |
| 3-Cyclohexene-1-carboxaldehyde | - | 1490 | MS | ND | 53.88 ± 5.14 c | 70.71 ± 5.38 c | 102.03 ± 7.33 b | 132.64 ± 9.09 a |
| (E)-2-Hexenol | 849 | 853 | MS + LRI | ND | 32.23 ± 1.96 b | 55.32 ± 1.36 a | 66.49 ± 44.86 a | ND |
| 1-Hexanol | 890 | 891 | MS + LRI | 34.25 ± 3.26 b | 56.46 ± 5.36 a | 40.36 ± 6.36 b | ND | ND |
| 1-Heptanol | 969 | 973 | MS + LRI | 106.35 ± 5.17 d | 362.92 ± 8.46 c | 373.9 ± 16.01 c | 552.42 ± 10.63 b | 621.57 ± 16.02 a |
| 1-Octen-3-ol | 981 | 984 | MS + LRI | 146.83 ± 8.8 b | 256.14 ± 8.49 a | 259.8 ± 17.27 a | 259.24 ± 11.87 a | ND |
| 1-Nonanol | 1150 | 1152 | MS + LRI | ND | 62.33 ± 6.32 b | 82.69 ± 3.95 a | 88.33 ± 6.33 a | ND |
| 3-methyl-6-ethyl-5-octen-1-ol | - | 1163 | MS | ND | ND | 133.48 ± 9.69 | ND | ND |
| 2-Propylcyclohexanol | - | 1264 | MS | ND | ND | ND | 64.64 ± 2.34 | 107.55 ± 7.41 |
| (E)-2-decen-1-ol | - | 1250 | MS | ND | 45.69 ± 6.69 a | 70.93 ± 2.11 a | 52.36 ± 3.98 a | 38.21 ± 1.80 a |
| Pentanol | 762 | 760 | MS + LRI | 62.36 ± 6.56 d | 91.33 ± 7.32 c | 99.22 ± 6.96 c | 143.63 ± 9.65 b | 156.26 ± 8.65 a |
| Cyclohexanol,3,5-dimethyl- | - | 970 | MS | ND | ND | ND | ND | 272.69 ± 8.79 |
| 2-Methylcyclopentanone | 846 | 850 | MS + LRI | ND | ND | 559.00 ± 17.1 | ND | ND |
| 2,3-Octandeione | 986 | 990 | MS + LRI | 132.25 ± 5.23 d | 231.96 ± 7.36 c | 553.36 ± 25.33 a | 423.36 ± 7.32 b | 134.36 ± 6.00 d |
| 2-Nonanone | 1089 | 1092 | MS + LRI | ND | ND | ND | 63.77 ± 7.66 | 171.66 ± 7.69 |
| 2-Decanone | 1194 | 1198 | MS + LRI | ND | ND | 63.91 ± 0.90 c | 157.27 ± 80.66 b | 310.12 ± 17.26 a |
| 2-Undecanone | 1296 | 1300 | MS + LRI | ND | ND | ND | 106.82 ± 7.99 | 217.96 ± 8.15 |
| 2-Tridecanone | 1498 | 1500 | MS + LRI | ND | ND | ND | 30.19 ± 2.37 | 49.30 ± 4.73 |
| 2,3-dimethyl-2-cyclopenten-1-one | 1035 | 1040 | MS + LRI | ND | ND | 694.55 ± 11.56 a | 119.71 ± 12.88 b | 63.41 ± 3.00 c |
| 2-Cyclopenten-1-one,2-butyl-3-methyl- | - | 979 | MS | ND | 75.74 ± 8.90 b | 141.99 ± 9.41 a | 80.36 ± 6.33 b | 39.96 ± 6.26 c |
| Ethyl hexanoate | 998 | 1000 | MS + LRI | 134.20 ± 8.35 d | 199.84 ± 8.83 c | 226.51 ± 12.23 b | 379.16 ± 8.92 a | 350.14 ± 7.36 a |
| γ-Caprolactone | 1055 | 1058 | MS + LRI | ND | ND | 264.16 ± 10.24 a | 134.52 ± 7.88 b | 64.55 ± 5.90 c |
| Ethyl heptanoate | 1097 | 1100 | MS + LRI | 28.17 ± 2.98 d | 66.69 ± 9.25 c | 243.41 ± 9.68 b | 303.13 ± 10.10 a | ND |
| Ethyl caprylate | 1199 | 1200 | MS + LRI | 32.66 ± 5.20 d | 50.28 ± 8.20 c | 94.95 ± 8.34 b | 147.70 ± 9.35 a | ND |
| Ethyl caprate | 1397 | 1400 | MS + LRI | ND | ND | 40.05 ± 5.37 a | 32.26 ± 7.36 ab | 15.21 ± 5.23 c |
| 5-Butyldihydro-2(3H)-furanone | 1260 | 1263 | MS + LRI | 172.65 ± 8.79 a | 168.25 ± 9.54 a | 165.13 ± 8.40 a | 83.94 ± 8.48 b | 70.36 ± 7.41 c |
| 4-Pentenoic acid ethyl ester | - | 1403 | MS | ND | ND | 100.72 ± 9.11 | 65.90 ± 5.47 | ND |
| 1-Decene | 989 | 992 | MS + LRI | ND | ND | 197.02 ± 8.48 c | 235.26 ± 8.89 b | 413.73 ± 13.09 a |
| Undecane | 1100 | 1103 | MS + LRI | 34.87 ± 5.83 a | 60.45 ± 4.83 a | 67.84 ± 6.49 a | 73.75 ± 9.50 a | 130.01 ± 15.24 a |
| Dodecane | 1200 | 1204 | MS + LRI | 68.89 ± 9.28 c | 98.82 ± 5.93 b | 207.11 ± 5.40 a | ND | ND |
| Tridecane | 1300 | 1301 | MS + LRI | 11.49 ± 2.48 e | 36.25 ± 2.36 d | 66.96 ± 5.96 c | 87.88 ± 4.47 b | 127.14 ± 12.63 a |
| 3-Methyltridecane | 1371 | 1373 | MS + LRI | ND | 82.60 ± 67.70 | ND | ND | ND |
| 2,6,10-Trimethyldodecane | 1376 | 1380 | MS + LRI | ND | 81.45 ± 11.60 c | 252.42 ± 11.91 a | 207.31 ± 5.71 b | 51.90 ± 5.44 d |
| 1-Tetradecene | 1396 | 1400 | MS + LRI | ND | ND | 337.85 ± 19.89 b | 356.20 ± 8.21 b | 437.06 ± 28.48 a |
| Tetradecane | 1400 | 1402 | MS + LRI | 9.95 ± 0.43 d | 68.02 ± 9.59 c | 99.89 ± 8.50 a | 85.76 ± 16.42 b | 70.99 ± 6.60 c |
| Hexadecane | 1600 | 1605 | MS + LRI | 4.57 ± 1.41 c | 7.93 ± 2.60 c | 31.56 ± 9.36 b | 39.22 ± 55.01 ab | 43.64 ± 6.50 a |
| Nonadecane | 1900 | 1901 | MS + LRI | ND | ND | 121.53 ± 8.23a | 84.57 ± 43.95 b | 24.13 ± 4.88 c |
| Oxirane, 2-octyl- | - | 1264 | MS + LRI | ND | ND | ND | ND | 93.15 ± 7.76 |
| 2,7-Dimethyloctane | - | 1172 | MS + LRI | ND | ND | ND | ND | 32.03 ± 7.38 |
| ( ± )-Limonene | - | 1081 | MS + LRI | 307.89 ± 7.53 e | 398.55 ± 9.33 d | 540.20 ± 17.41 c | 687.57 ± 13.56 b | 917.28 ± 9.11 a |
| 3,5-Dimethyl-1-Hexene | - | 1639 | MS + LRI | ND | ND | ND | ND | 248.17 ± 12.26 |
| Toluene | 757 | 760 | MS + LRI | 127.40 ± 9.75 b | 129.56 ± 7.83 b | 244.88 ± 12.00 a | 257.23 ± 6.11 a | 102.35 ± 12.96 c |
| 2,5-Dimethylpyrazine | 917 | 920 | MS + LRI | ND | ND | ND | ND | 586.28 ± 15.37 |
| 2-Amylfuran | 991 | 993 | MS + LRI | 47.87 ± 7.82 | ND | ND | ND | ND |
| cis-Anethol | 1286 | 1290 | MS + LRI | 42.93 ± 9.78 d | 45.90 ± 4.09 cd | 56.61 ± 6.05 c | 84.66 ± 8.96 b | 127.83 ± 9.02 a |
| 2-Ethyl-3,5-dimethylpyrazine | - | 1302 | MS + LRI | 243.59 ± 30.29 a | 120.11 ± 3.99 b | 87.87 ± 9.82 c | 70.11 ± 3.92 d | 60.11 ± 3.92 e |
| 2-butyl tetrahydrofuran | - | 1325 | MS + LRI | 155.36 ± 11.52 a | 150.36 ± 8.27 a | 140.46 ± 10.25 a | 74.71 ± 6.68 b | 57.96 ± 7.34 b |
| 2-Pentylpyridine | - | 1453 | MS + LRI | ND | ND | ND | ND | 32.32 ± 9.76 |
| 2,5-Dimethyltetrahydrofuran | - | 1520 | MS + LRI | 32.54 ± 4.32 | ND | ND | ND | ND |
| Volatile Compounds | LRI * | Identification + | F0L4 | F1L3 | F2L2 | F3L1 | |
|---|---|---|---|---|---|---|---|
| Literature | Calculated | ||||||
| Hexanal | 800 | 802 | MS + LRI | 91.32 ± 10.97 c | 237.98 ± 12.33 b | 378.81 ± 10.00 a | 397.18 ± 11.26 a |
| Heptanal | 901 | 904 | MS + LRI | 69.14 ± 5.23 c | 232.18 ± 2.17 b | 351.09 ± 16.74 a | 374.97 ± 12.63 a |
| Octanal | 1004 | 1005 | MS + LRI | 63.13 ± 2.42 c | 160.22 ± 7.29 b | 264.52 ± 12.96 a | 280.03 ± 18.29 a |
| Nonanal | 1112 | 1114 | MS + LRI | 94.62 ± 10.03 c | 181.54 ± 11.6 b | 206.70 ± 16.03 a | 213.49 ± 12.64 a |
| Decanal | 1202 | 1205 | MS + LRI | 121.43 ± 6.47 c | 123.90 ± 9.11 c | 143.39 ± 9.55 b | 277.83 ± 11.08 a |
| (2E)-2-Decenal | 1265 | 1268 | MS + LRI | ND | 29.04 ± 1.86 b | 50.34 ± 2.03 a | 54.06 ± 1.98 a |
| 7-Hydroxy-3,7-dimethyloctanal | 1300 | 1303 | MS + LRI | ND | ND | ND | 118.10 ± 4.03 a |
| Ethyl hexanoate | 998 | 1000 | MS + LRI | ND | 63.49 ± 2.65 c | 103.54 ± 1.61 a | 94.32 ± 2.31 b |
| γ-Caprolactone | 1055 | 1058 | MS + LRI | ND | ND | 74.15 ± 4.01 a | 57.32 ± 1.51 b |
| (±)-Limonene | - | 1081 | MS | 75.53 ± 3.75 c | 183.83 ± 6.24 b | 205.24 ± 6.98 a | 207.29 ± 6.01 a |
| Undecane | 1100 | 1103 | MS + LRI | ND | 66.67 ± 2.36 b | 80.33 ± 1.87 a | 82.14 ± 3.35 a |
| Dodecane | 1200 | 1204 | MS + LRI | ND | 60.77 ± 4.36 | ND | ND |
| 2,6,10-Trimethyldodecane | 1376 | 1380 | MS + LRI | ND | ND | 48.34 ± 3.02 b | 63.75 ± 0.80 a |
| 1-Tetradecene | 1396 | 1400 | MS + LRI | ND | ND | 41.29 ± 1.12 | ND |
| Tetradecane | 1400 | 1402 | MS + LRI | ND | ND | 61.58 ± 4.43 a | 49.34 ± 4.54 b |
| Hexadecane | 1600 | 1605 | MS + LRI | ND | 46.42 ± 2.15 c | 74.02 ± 6.26 b | 97.00 ± 0.02 a |
| 2,5-Dimethylpyrazine | 917 | 920 | MS + LRI | 32.98 ± 0.18 b | ND | ND | 118.10 ± 4.03 a |
| 1-Heptanol | 969 | 973 | MS + LRI | ND | ND | 33.40 ± 2.55 | 36.15 ± 3.11 |
| P-Xylene | 875 | 880 | MS + LRI | ND | ND | 112.55 ± 36.38 | ND |
| cis-Anethol | 1286 | 1290 | MS + LRI | 62.17 ± 2.32 c | 141.35 ± 5.33 b | 201.82 ± 12.44 a | 196.00 ± 8.93 a |
| 2-Ethyl-3,5-dimethylpyrazine | - | 1302 | MS + LRI | 33.69 ± 2.31 c | 93.15 ± 5.75 ab | 87.93 ± 2.81 b | 97.29 ± 4.88 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, M.; Bai, F.; Yao, W.; You, L.; Liu, D. Effect of Fat to Lean Meat Ratios on the Formation of Volatile Compounds in Mutton Shashliks. Foods 2023, 12, 1929. https://doi.org/10.3390/foods12101929
Zhang M, Li M, Bai F, Yao W, You L, Liu D. Effect of Fat to Lean Meat Ratios on the Formation of Volatile Compounds in Mutton Shashliks. Foods. 2023; 12(10):1929. https://doi.org/10.3390/foods12101929
Chicago/Turabian StyleZhang, Mingcheng, Mingyang Li, Fangfang Bai, Wensheng Yao, Litang You, and Dengyong Liu. 2023. "Effect of Fat to Lean Meat Ratios on the Formation of Volatile Compounds in Mutton Shashliks" Foods 12, no. 10: 1929. https://doi.org/10.3390/foods12101929