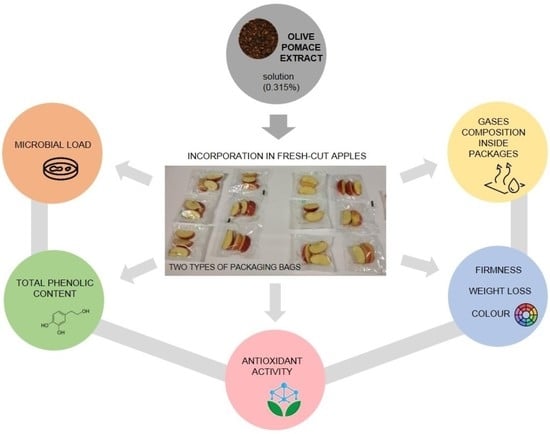
Effect of Olive Pomace Extract Application and Packaging Material on the Preservation of Fresh-Cut Royal Gala Apples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Olive Pomace Samples and Irradiation Experiments
2.3. Olive Pomace Natural Ingredients: Phenolic Extract Preparation
2.4. Polylactic Acid (PLA) and Oriented Polypropylene (OPP) Films Packages
2.5. Preparation of Fresh-Cut Apples
2.6. Analytical Methods
2.6.1. Soluble Solids Content, Titratable Acidity and Respiration Rate
2.6.2. Composition of the Atmosphere Inside the Packages
2.6.3. Weight Loss, Firmness and Surface Color
2.6.4. Microbial Load
2.6.5. Total Phenolic Content and Antioxidant Activity
2.7. Statistical Analysis
2.8. Principal Components and Overlayed K-Means Clustering
3. Results and Discussion
3.1. Physicochemical Characterization of the Samples
3.2. Composition of the Atmosphere Inside the Package
3.3. Weight Loss, Firmness and Surface Color
3.4. Microbial Load
3.5. Total Phenolic Content and Antioxidant Activity
3.6. Principal Component Analysis and Clustering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guarrasi, V.; Giacomazza, D.; Germanà, M.A.; Amenta, M.; San Biaggio, P.L. Monitoring the Shelf-Life of Minimally Processed Fresh-Cut Apple Slices By Physical Chemical Analysis and Electronic Nose. Agrotechnology 2014, 3, 126. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatística Consumo Humano de Frutos per Capita (Kg/Hab) Por Especie Frutícola (Balanços de Mercado). Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0000163&contexto=bd&selTab=tab2 (accessed on 20 April 2023).
- Solís-Contreras, G.A.; Rodríguez-Guillermo, M.C.; de la Luz Reyes-Vega, M.; Aguilar, C.N.; Rebolloso-Padilla, O.N.; Corona-Flores, J.; de Abril Alexandra Soriano-Melgar, L.; Ruelas-Chacon, X. Extending Shelf-Life and Quality of Minimally Processed Golden Delicious Apples with Three Bioactive Coatings Combined with Cinnamon Essential Oil. Foods 2021, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic Browning of Fruit and Vegetables: A Review. In Enzymes in Food Technology; Kuddus, M., Ed.; Springer: Singapore, 2018; pp. 63–78. [Google Scholar]
- Lee, C.Y.; Smith, N.L. Minimal Processing of New York Apples. N. Y. Food Life Sci. Bull. 1995, 145, 1–11. [Google Scholar]
- Hamdan, N.; Lee, C.H.; Wong, S.L.; Fauzi, C.E.N.C.A.; Zamri, N.M.A.; Lee, T.H. Prevention of Enzymatic Browning by Natural Extracts and Genome-Editing: A Review on Recent Progress. Molecules 2022, 27, 1101. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.I.; Saleh, M.A. Browning Inhibition Mechanisms by Cysteine, Ascorbic Acid and Citric Acid, and Identifying PPO-Catechol-Cysteine Reaction Products. J. Food Sci. Technol. 2015, 52, 3651–3659. [Google Scholar] [CrossRef]
- Grispoldi, L.; Karama, M.; El-Ashram, S.; Saraiva, C.; García-Díez, J.; Chalias, A.; De Gennis, M.; Vannuccini, A.; Poerio, G.; Torlai, P.; et al. A Study on the Application of Natural Extracts as Alternatives to Sodium Nitrite in Processed Meat. J. Food Process. Preserv. 2022, 46, e16351. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.; Ferreira, I.C.F.R. A Comparative Study between Natural and Synthetic Antioxidants: Evaluation of Their Performance after Incorporation into Biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Barros, L.; Barreira, J.C.M.; Antonio, A.L.; Beatriz, M.; Oliveira, P.P.; Filomena Barreiro, M.; Ferreira, I.C.F.R. Cottage Cheeses Functionalized with Fennel and Chamomile Extracts: Comparative Performance between Free and Microencapsulated Forms. Food Chem. 2016, 199, 720–726. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Carocho, M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Fortification of Yogurts with Different Antioxidant Preservatives: A Comparative Study between Natural and Synthetic Additives. Food Chem. 2016, 210, 262–268. [Google Scholar] [CrossRef]
- Madureira, J.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Barros, L.; Santos-Buelga, C.; Margaça, F.M.A.; Ferreira, I.C.F.R.; Cabo Verde, S. The Use of Gamma Radiation for Extractability Improvement of Bioactive Compounds in Olive Oil Wastes. Sci. Total Environ. 2020, 727, 138706. [Google Scholar] [CrossRef]
- Madureira, J.; Albuquerque, B.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Santos-Buelga, C.; Margaça, F.M.A.; Ferreira, I.C.F.R.; Cabo Verde, S.; Barros, L. Ultrasound-Assisted Extraction of Hydroxytyrosol and Tyrosol from Olive Pomace Treated by Gamma Radiation: Process Optimization and Bioactivity Assessment. Food Funct. 2023, 14, 3038–3050. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Control of Microbial Growth in Bakery Products Fortified with Polyphenols Recovered from Olive Mill Wastewater. Environ. Technol. Innov. 2018, 10, 1–15. [Google Scholar] [CrossRef]
- Saleh, E.; Morshdy, A.E.; El-Manakhly, E.; Al-Rashed, S.; Hetta, H.F.; Jeandet, P.; Yahia, R.; El-Saber Batiha, G.; Ali, E. Effects of Olive Leaf Extracts as Natural Preservative on Retailed Poultry Meat Quality. Foods 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal Foods Fortified with by-Products from the Olive Oil Industry. Food Biosci. 2020, 33, 100490. [Google Scholar] [CrossRef]
- Lin, S.; Chi, W.; Hu, J.; Pan, Q.; Zheng, B.; Zeng, S. Sensory and Nutritional Properties of Chinese Olive Pomace Based High Fibre Biscuit. Emirates J. Food Agric. 2017, 29, 495–501. [Google Scholar] [CrossRef]
- Durante, M.; Bleve, G.; Selvaggini, R.; Veneziani, G.; Servili, M.; Mita, G. Bioactive Compounds and Stability of a Typical Italian Bakery Products “Taralli” Enriched with Fermented Olive Paste. Molecules 2019, 24, 3258. [Google Scholar] [CrossRef]
- Cecchi, L.; Schuster, N.; Flynn, D.; Bechtel, R.; Bellumori, M.; Innocenti, M.; Mulinacci, N.; Guinard, J.X. Sensory Profiling and Consumer Acceptance of Pasta, Bread, and Granola Bar Fortified with Dried Olive Pomace (Pâté): A Byproduct from Virgin Olive Oil Production. J. Food Sci. 2019, 84, 2995–3008. [Google Scholar] [CrossRef]
- Madureira, J.; Margaça, F.M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Verde, S.C.; Barros, L. Applications of Bioactive Compounds Extracted from Olive Industry Wastes: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 453–476. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional Compounds from Olive Pomace to Obtain High-Added Value Foods—A Review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and Challenges in the Development of Bio-Based Barrier Coating Materials for Paper/Cardboard Food Packaging; a Review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Madureira, J.; Pimenta, A.I.; Popescu, L.; Besleaga, A.; Dias, M.I.; Santos, P.M.P.; Melo, R.; Ferreira, I.C.F.R.; Cabo Verde, S.; Margaça, F.M.A. Effects of Gamma Radiation on Cork Wastewater: Antioxidant Activity and Toxicity. Chemosphere 2017, 169, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, D.; Madureira, J.; Silva, T.; Melo, R.; Santos, P.M.P.; Ferreira, A.; Trigo, M.J.; Falcão, A.N.; Margaça, F.M.A.; Cabo Verde, S. Post-Harvest Treatment of Cherry Tomatoes by Gamma Radiation: Microbial and Physicochemical Parameters Evaluation. Innov. Food Sci. Emerg. Technol. 2016, 36, 1–9. [Google Scholar] [CrossRef]
- Elias, M.I.; Madureira, J.; Santos, P.M.P.; Carolino, M.M.; Margaça, F.M.A.; Verde, S.C. Preservation Treatment of Fresh Raspberries by E-Beam Irradiation. Innov. Food Sci. Emerg. Technol. 2020, 66, 102487. [Google Scholar] [CrossRef]
- Barkaoui, S.; Mankai, M.; Miloud, N.B.; Kraïem, M.; Madureira, J.; Verde, S.C.; Boudhrioua, N. E-Beam Irradiation of Strawberries: Investigation of Microbiological, Physicochemical, Sensory Acceptance Properties and Bioactive Content. Innov. Food Sci. Emerg. Technol. 2021, 73, 102769. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Dueñas, M.; Antonio, A.L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Gamma Irradiation Improves the Extractability of Phenolic Compounds in Ginkgo biloba L. Ind. Crops Prod. 2015, 74, 144–149. [Google Scholar] [CrossRef]
- Whittaker, B.; Watts, M.F. The Influence of Dose Rate, Ambient Temperature and Time on the Radiation Response of Harwell PMMA Dosimeters. Radiat. Phys. Chem. 2001, 60, 101–110. [Google Scholar] [CrossRef]
- Madureira, J.; Melgar, B.; Santos-Buelga, C.; Margaça, F.M.A.; Ferreira, I.C.F.R.; Barros, L.; Verde, S.C. Phenolic Compounds from Irradiated Olive Wastes: Optimization of the Heat-Assisted Extraction Using Response Surface Methodology. Chemosensors 2021, 9, 231. [Google Scholar] [CrossRef]
- Vieira, T.M.; Moldão-Martins, M.; Alves, V.D. Composite Coatings of Chitosan and Alginate Emulsions with Olive Oil to Enhance Postharvest Quality and Shelf Life of Fresh Figs (Ficus carica L. Cv. ’Pingo De Mel’). Foods 2021, 10, 718. [Google Scholar] [CrossRef]
- Vieira, T.M.; Alves, V.D.; Moldão Martins, M. Application of an Eco-Friendly Antifungal Active Package to EXtend the Shelf Life of Fresh Red Raspberry (Rubus idaeus L. Cv. ’Kweli’). Foods 2022, 11, 1805. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Barkaoui, S.; Madureira, J.; Santos, P.M.P.; Margaça, F.M.A.; Miloud, N.B.; Mankai, M.; Boudhrioua, N.M.; Cabo Verde, S. Effect of Ionizing Radiation and Refrigeration on the Antioxidants of Strawberries. Food Bioprocess Technol. 2020, 13, 1516–1527. [Google Scholar] [CrossRef]
- Jha, S.N.; Rai, D.R.; Shrama, R. Physico-Chemical Quality Parameters and Overall Quality Index of Apple during Storage. J. Food Sci. Technol. 2012, 49, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Ghafir, S.A.M.; Gadalla, S.O.; Murajei, B.N.; El-Nady, M.F. Physiological and Anatomical Comparison between Four Different Apple Cultivars under Cold-Storage Conditions. Acta Biol. Szeged. 2009, 53, 21–26. [Google Scholar]
- Shyu, Y.S.; Chen, G.W.; Chiang, S.C.; Sung, W.C. Effect of Chitosan and Fish Gelatin Coatings on Preventing the Deterioration and Preserving the Quality of Fresh-Cut Apples. Molecules 2019, 24, 2008. [Google Scholar] [CrossRef]
- Jan, I.; Rab, A.; Sajid, M.; Ali, A.; Shah, S.T. Response of Apple Cultivars To Different Storage Durations. Sarhad J. Agric 2012, 28, 219–225. [Google Scholar]
- Rydzak, L.; Kobus, Z.; Nadulski, R.; Wilczyński, K.; Pecyna, A.; Santoro, F.; Sagan, A.; Starek-Wójcicka, A.; Krzywicka, M. Analysis of Selected Physicochemical Properties of Commercial Apple Juices. Processes 2020, 8, 1457. [Google Scholar] [CrossRef]
- Muhammad, A.; Ayub, M.; Zeb, A. Physicochemical Analysis of Apple Pulp from Mashaday Variety during Storage. Agric. Biol. J. N. Am. 2011, 2, 192–196. [Google Scholar] [CrossRef]
- Rocha, A.M.C.N.; Morais, A.M.M.B. Shelf Life of Minimally Processed Apple (Cv. Jonagored) Determined by Colour Changes. Food Control 2003, 14, 13–20. [Google Scholar] [CrossRef]
- Rojas-Grau, M.A.; Sobrino-López, A.; Tapia, M.S.; Martín-Belloso, O. Browning Inhibition in Fresh-Cut ‘ Fuji ’ Apple Slices by Natural Antibrowning Agents. J. Food Sci. 2006, 71, 59–65. [Google Scholar] [CrossRef]
- González-Buesa, J.; Page, N.; Kaminski, C.; Ryser, E.T.; Beaudry, R.; Almenar, E. Effect of Non-Conventional Atmospheres and Bio-Based Packaging on the Quality and Safety of Listeria Monocytogenes-Inoculated Fresh-Cut Celery (Apium graveolens L.) during Storage. Postharvest Biol. Technol. 2014, 93, 29–37. [Google Scholar] [CrossRef]
- Zhou, H.; Kawamura, S.; Koseki, S.; Kimura, T. Comparative Quality Changes of Fresh-Cut Melon in Bio-Based and Petroleum-Based Plastic Containers during Storage. Environ. Control Biol. 2016, 54, 93–99. [Google Scholar] [CrossRef]
- Song, H.Y.; Jo, W.S.; Song, N.B.; Min, S.C.; Song, K. Bin Quality Change of Apple Slices Coated with Aloe Vera Gel during Storage. J. Food Sci. 2013, 78, C817–C822. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Varghese, E. Improving the Shelf Life of Fresh-Cut ‘Royal Delicious’ Apple with Edible Coatings and Anti-Browning Agents. J. Food Sci. Technol. 2018, 55, 3767–3778. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.R.; Tomadoni, B.; Martín-Belloso, O.; Soliva-Fortuny, R. Preservation of Fresh-Cut Apple Quality Attributes by Pulsed Light in Combination with Gellan Gum-Based Prebiotic Edible Coatings. LWT-Food Sci. Technol. 2015, 64, 1130–1137. [Google Scholar] [CrossRef]
- Osuga, R.; Koide, S.; Sakurai, M.; Orikasa, T.; Uemura, M. Quality and Microbial Evaluation of Fresh-Cut Apples during 10 Days of Supercooled Storage. Food Control 2021, 126, 108014. [Google Scholar] [CrossRef]
- Graça, A.; Santo, D.; Esteves, E.; Nunes, C.; Abadias, M. Evaluation of Microbial Quality and Yeast Diversity in Fresh-Cut Apple. Food Microbiol. 2015, 51, 179–185. [Google Scholar] [CrossRef]
- Botondi, R.; Bartoloni, S.; Baccelloni, S.; Mencarelli, F. Biodegradable PLA (Polylactic Acid) Hinged Trays Keep Quality of Fresh-Cut and Cooked Spinach. J. Food Sci. Technol. 2015, 52, 5938–5945. [Google Scholar] [CrossRef]
- Almenar, E.; Samsudin, H.; Auras, R.; Harte, B.; Rubino, M. Postharvest Shelf Life Extension of Blueberries Using a Biodegradable Package. Food Chem. 2008, 110, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Sarengaowa, W.; Guan, Y.; Feng, K. Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef] [PubMed]





| L* | |||||||
|---|---|---|---|---|---|---|---|
| T0 | T2 | T5 | T7 | T9 | T12 | ||
| OPP | EXT | 79.7 ± 0.2 a,A | 77.09 ± 0.47 b,B | 74.3 ± 0.3 c,C | 74.9 ± 0.4 c,C | 74.04 ± 0.47 c,C | 74.3 ± 0.5 c,C |
| AA | 79.4 ± 0.2 a,A | 78.6 ± 0.3 a,b,A | 77.4 ± 0.3 b,c,B | 76.9 ± 0.4 c,B | 77.2 ± 0.4 c,B | 76.8 ± 0.3 c,B | |
| PLA | EXT | 79.7 ± 0.2 a,A | 76.97 ± 0.22 b,c,B | 76.1 ± 0.5 c,B | 77.3 ± 0.3 b,c,A,B | 77.6 ± 0.3 b,A,B | 76.7 ± 0.3 b,c,B |
| AA | 79.4 ± 0.2 a,A | 78.5 ± 0.3 a,A | 78.9 ± 0.2 a,A | 78.5 ± 0.2 a,A | 78.6 ± 0.3 a,A | 78.7 ± 0.3 a,A | |
| C | |||||||
| T0 | T2 | T5 | T7 | T9 | T12 | ||
| OPP | EXT | 20.9 ± 0.2 c,A | 29.1 ± 0.6 b,A | 28.6 ± 0.4 b,A | 29.4 ± 0.5 b,A | 32.2 ± 0.5 a,A | 32.1 ± 0.4 a,A |
| AA | 19.7 ± 0.2 c,B | 24.9 ± 0.5 a,b,B | 22.9 ± 0.5 b,C | 24.1 ± 0.5 a,b,C | 23.5 ± 0.5 b,C | 25.3 ± 0.4 a,C | |
| PLA | EXT | 20.9 ± 0.2 b,A | 26.6 ± 0.5 a,B | 25.8 ± 0.5 a,B | 25.8 ± 0.4 a,B | 26.1 ± 0.3 a,B | 27.01 ± 0.40 a,B |
| AA | 19.7 ± 0.2 d,B | 25.4 ± 0.5 a,B | 23.2 ± 0.4 b,c,C | 22.8 ± 0.3 c,C | 23.1 ± 0.4 b,c,C | 24.5 ± 0.4 a,b,C | |
| h° | |||||||
| T0 | T2 | T5 | T7 | T9 | T12 | ||
| OPP | EXT | 99.3 ± 0.1 a,A | 87.6 ± 0.4 c,D | 89.3 ± 0.3 b,C | 89.4 ± 0.4 b,C | 89.3 ± 0.3 b,C | 87.9 ± 0.3 b,c,B |
| AA | 99.6 ± 0.3 a,A | 93.4 ± 0.4 b,B | 93.5 ± 0.4 b,A | 92.7 ± 0.4 b,A | 92.6 ± 0.4 b,A | 92.7 ± 0.3 b,A | |
| PLA | EXT | 99.3 ± 0.1 a,A | 91.6 ± 0.3 b,C | 91.5 ± 0.3 b,B | 90.7 ± 0.2 b,B | 90.7 ± 0.3 b,B | 89.5 ± 0.4 c,B |
| AA | 99.6 ± 0.3 a,A | 95.8 ± 0.4 b,A | 94.7 ± 0.3 b,c,A | 93.6 ± 0.2 c,d,A | 93.5 ± 0.3 c,d,A | 93.1 ± 0.4 d,A | |
| ΔE | |||||||
| T0 | T2 | T5 | T7 | T9 | T12 | ||
| OPP | EXT | - | 11.4 ± 0.7 a,b,A | 10.3 ± 0.5 b,A | 11.8 ± 0.4 a,b,A | 11.8 ± 0.7 a,b,A | 14.1 ± 0.7 a,A |
| AA | - | 5.1 ± 0.6 a,B | 4.3 ± 0.5 a,C | 5.4 ± 0.6 a,C,B | 5.2 ± 0.5 a,B,C | 6.4 ± 0.5 a,B,C | |
| PLA | EXT | - | 7.07 ± 0.47 a,B | 7.4 ± 0.7 a,B | 6.3 ± 0.5 a,B | 6.7 ± 0.3 a,B | 8.02 ± 0.46 a,B |
| AA | - | 5 ± 1 a,B | 4.2 ± 0.5 a,C | 4.01 ± 0.30 a,C | 4.7 ± 0.6 a,C | 5.5 ± 0.5 a,C | |
| Mesophilic Bacteria (Log CFU/g) | Filamentous Fungi (Log CFU/g) | Coliforms (Log CFU/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T5 | T12 | T0 | T5 | T12 | T0 | T5 | T12 | ||
| OPP | EXT | 3.5 ± 0.1 b,B | 3.4 ± 0.1 b,D | 5.2 ± 0.1 a,A | 2.5 ± 0.1 c,C | 3.3 ± 0.1 b,C | 5.25 ± 0.05 a,A | n.d. | n.d. | n.d. |
| AA | 3.2 ± 0.1 c,B,C | 4.3 ± 0.1 b,B | 5.6 ± 0.1 a,A | 3.2 ± 0.1 c,B | 4.03 ± 0.09 b,B | 5.25 ± 0.04 a,A | n.d. | 2.0 ± 0.3 b,A | 3.1 ± 0.1 a,A | |
| NT | 3.3 ± 0.1 c,B,C | 5.2 ± 0.1 b,A | 5.7 ± 0.1 a,A | 2.9 ± 0.2 c,B,C | >6 a,A | 5.18 ± 0.04 b,A | 2.2 ± 0.1 a | 2.2 ± 0.1 a,A | n.d. | |
| PLA | EXT | 2.9 ± 0.1 b,B,C | 2.4 ± 0.1 c,E | 3.75 ± 0.03 a,C | 2.4 ± 0.1 b,C | 2.44 ± 0.05 b,D | 3.3 ± 0.1 a,C | n.d. | n.d. | n.d. |
| AA | 2.9 ± 0.1 c,C | 3.90 ± 0.04 b,C | 4.2 ± 0.1 a,B | 2.8 ± 0.3 b,B,C | 3.84 ± 0.03 a,B | 4.2 ± 0.2 a,B | n.d. | 1.8 ± 0.1 a,A | 1.6 ± 0.2 a,B | |
| NT | 4.63 ± 0.03 b,A | 4.5 ± 0.1 b,B | 5.80 ± 0.01 a,A | 4.44 ± 0.05 b,A | 4.02 ± 0.07 c,B | 5.3 ± 0.1 a,A | n.d. | 1.5 ± 0.1 a,A | 1.3 ± 0.3 a,B | |
| Mg GAE/g Extract | |||||||
|---|---|---|---|---|---|---|---|
| T0 | T2 | T5 | T7 | T9 | T12 | ||
| OPP | EXT | 5.4 ± 0.2 a,b,c,A | 4.9 ± 0.2 b,c,A | 4.6 ± 0.1 c,A,B | 4.7 ± 0.1 b,c,A | 5.4 ± 0.2 a,b,A | 6.1 ± 0.3 a,A,B |
| AA | 5.7 ± 0.4 b,A | 4.8 ± 0.2 b,c,A | 4.4 ± 0.2 c,B | 4.5 ± 0.2 c,A | 5.1 ± 0.3 b,c,A | 7.0 ± 0.2 a,A | |
| NT | 4.68 ± 0.07 a,b,A | 3.5 ± 0.1 c,B | 4.08 ± 0.15 b,c,B | 4.42 ± 0.01 a,b,c,A,B | 5.01 ± 0.22 a,b,A | 5.4 ± 0.5 a,B | |
| PLA | EXT | 4.9 ± 0.4 a,b,A | 5.0 ± 0.2 a,b,A | 4.3 ± 0.1 b,c,B | 3.8 ± 0.1 c,B | 4.9 ± 0.2 a,b,A | 5.8 ± 0.3 a,B |
| AA | 5.0 ± 0.2 b,A | 4.6 ± 0.2 b,A,B | 5.3 ± 0.2 b,A | 5.0 ± 0.2 b,A | 5.04 ± 0.15 b,A | 6.5 ± 0.2 a,A,B | |
| NT | 4.5 ± 0.3 b,c,A | 3.70 ± 0.01 d,A,B | 4.48 ± 0.07 b,c,A,B | 5.01 ± 0.00 a,b,A | 4.14 ± 0.06 c,d,A | 5.53 ± 0.09 a,B | |
| mmol FSE/g Extract | |||||||
|---|---|---|---|---|---|---|---|
| T0 | T2 | T5 | T7 | T9 | T12 | ||
| OPP | EXT | 0.078 ± 0.004 b,A | 0.089 ± 0.006 a,b,A,,B | 0.098 ± 0.002 a,B | 0.091 ± 0.003 a,b,B | 0.079 ± 0.006 b,A | 0.092 ± 0.003 a,b,B,C |
| AA | 0.080 ± 0.005 c,A | 0.087 ± 0.003 b,c,A,B | 0.098 ± 0.003 a,b,B | 0.089 ± 0.003 b,c,B | 0.091 ± 0.005 a,b,c,A | 0.104 ± 0.001 a,A | |
| NT | 0.0756 ± 0.0001 c,A | 0.075 ± 0.003 c,A | 0.096 ± 0.003 a,B | 0.085 ± 0.002 b,B,C | 0.082 ± 0.002 b,c,A | 0.0862 ± 0.0004 b,C | |
| PLA | EXT | 0.076 ± 0.002 b,c,A | 0.096 ± 0.005 a,A | 0.093 ± 0.003 a,b,B | 0.075 ± 0.003 c,C | 0.082 ± 0.005 a,c,A | 0.096 ± 0.005 a,A,B,C |
| AA | 0.079 ± 0.002 d,A | 0.088 ± 0.003 c,d,A,B | 0.117 ± 0.003 a,A | 0.103 ± 0.001 b,A | 0.089 ± 0.002 c,A | 0.100 ± 0.001 b,A,B | |
| NT | 0.07623 ± 0.00005 c,A | 0.065 ± 0.003 d,B | 0.100 ± 0.001 a,B | 0.094 ± 0.003 a,b,A,B | 0.0683 ± 0.0004 c,d,A | 0.0890 ± 0.0001 b,B,C | |
| IC50 (mg/mL) | |||||||
| T0 | T2 | T5 | T7 | T9 | T12 | ||
| OPP | EXT | 6.3 ± 0.2 a,B | 4.3 ± 0.1 c,D | 4.0 ± 0.1 c,D | 5.3 ± 0.1 b,C | 4.3 ± 0.1 c,D | 6.4 ± 0.1 a,B,C |
| AA | 7.8 ± 0.2 a,A | 4.5 ± 0.1 c,C,D | 5.7 ± 0.1 b,B | 5.3 ± 0.2 b,C | 7.2 ± 0.2 a,A | 5.3 ± 0.2 b,D | |
| NT | 7.12 ± 0.04 a,b,A | 6.2 ± 0.1 c,d,B | 5.7 ± 0.2 d,B | 6.6 ± 0.1 b,c,A,B | 4.16 ± 0.05 e,D | 7.5 ± 0.1 a,A | |
| PLA | EXT | 7.4 ± 0.1 a,A | 4.7 ± 0.1 d,C | 6.8 ± 0.1 b,A | 7.4 ± 0.1 a,A | 5.7 ± 0.1 c,C | 7.0 ± 0.1 a,b,A,B |
| AA | 5.5 ± 0.1 a,C | 4.4 ± 0.1 b,C,D | 4.1 ± 0.1 b,C,D | 5.1 ± 0.1 a,C | 4.4 ± 0.1 b,D | 5.4 ± 0.2 a,D | |
| NT | 7.3 ± 0.1 b,A | 8.2 ± 0.1 a,A | 4.6 ± 0.1 e,C | 6.3 ± 0.1 c,B | 6.3 ± 0.2 c,B | 5.7 ± 0.1 d,C,D | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madureira, J.; Melgar, B.; Alves, V.D.; Moldão-Martins, M.; Margaça, F.M.A.; Santos-Buelga, C.; Barros, L.; Cabo Verde, S. Effect of Olive Pomace Extract Application and Packaging Material on the Preservation of Fresh-Cut Royal Gala Apples. Foods 2023, 12, 1926. https://doi.org/10.3390/foods12091926
Madureira J, Melgar B, Alves VD, Moldão-Martins M, Margaça FMA, Santos-Buelga C, Barros L, Cabo Verde S. Effect of Olive Pomace Extract Application and Packaging Material on the Preservation of Fresh-Cut Royal Gala Apples. Foods. 2023; 12(9):1926. https://doi.org/10.3390/foods12091926
Chicago/Turabian StyleMadureira, Joana, Bruno Melgar, Vítor D. Alves, Margarida Moldão-Martins, Fernanda M. A. Margaça, Celestino Santos-Buelga, Lillian Barros, and Sandra Cabo Verde. 2023. "Effect of Olive Pomace Extract Application and Packaging Material on the Preservation of Fresh-Cut Royal Gala Apples" Foods 12, no. 9: 1926. https://doi.org/10.3390/foods12091926