Exploring the Impact of α-Amylase Enzyme Activity and pH on Flavor Perception of Alcoholic Drinks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ascending Method of Limits for Primary Taste Detection Thresholds in Aqueous and Hydroalcoholic Solutions
2.2. Tasting Panel Characterization and Laboratory Conditions
2.3. Methodology for pH Changes and Enzyme Activity Evaluation
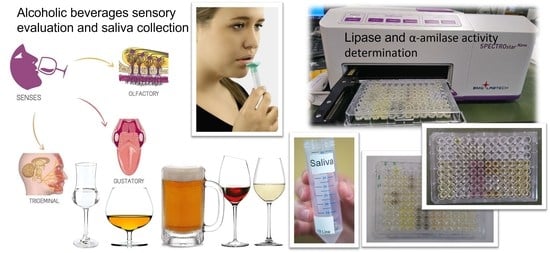
2.3.1. Solutions and Alcoholic Beverages
2.3.2. Tasting Procedure and Saliva Collection
2.3.3. Lipase and α-Amylase Activity Determination
2.4. Beverage Sensory Evaluation through a Descriptive Analysis (DA) Sensory Test
2.5. Data Analysis
3. Results
3.1. Primary Taste Detection Thresholds in Aqueous and Hydroalcoholic Solutions
3.2. Determination of Temperature and pH Changes and Enzyme Evaluation in Aqueous/Hydroalcoholic Solutions and Beverages
3.2.1. Temperature and pH
3.2.2. Lipase and α-Amylase Enzymes Activity
3.3. Sensory Profile of the Alcoholic Drinks Determined by Descriptive Analysis (DA) Sensory Test
4. Discussion
4.1. Sensation and Perception Threshold Determination
4.2. Influence of Saliva on the pH Variations
4.3. Enzymatic Activity Elicited by Aqueous and Hydroalcoholic Solutions and Alcoholic Beverages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Precone, V.; Beccari, T.; Stuppia, L.; Baglivo, M.; Paolacci, S.; Manara, E.; Miggiano, G.A.D.; Falsini, B.; Trifirò, A.; Zanlari, A.; et al. Taste, olfactory and texture related genes and food choices: Implications on health status. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1305–1321. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Bartolomé, B.; Moreno-Arribas, M.V. Mouthfeel perception of wine: Oral physiology, components, and instrumental characterization. Trends Food Sci. Technol. 2017, 59, 49–59. [Google Scholar] [CrossRef]
- Norton, V.; Lignou, S.; Methven, L. Influence of Age and Individual Differences on Mouthfeel Perception of Whey Protein-Fortified Products: A Review. Foods 2021, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.T.; Klein, A.H.; Carstens, E. Chemogenic Subqualities of Mouthfeel. Chem. Senses 2019, 44, 281–288. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Feron, G.; Canon, F. Main effects of human saliva on flavour perception and the potential contribution to food consumption. Proc. Nutr. Soc. 2018, 77, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Ployon, S.; Morzel, M.; Canon, F. The role of saliva in aroma release and perception. Food Chem. 2017, 226, 212–220. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. BioMed Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.; Neiers, F.; Feron, G.; Canon, F. The Relationship Between Salivary Redox, Diet, and Food Flavor Perception. Front. Nutr. 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Feron, G. Unstimulated saliva: Background noise in taste molecules. J. Texture Stud. 2019, 50, 6–18. [Google Scholar] [CrossRef] [Green Version]
- Martina, E.; Campanati, A.; Diotallevi, F.; Offidani, A. Saliva and Oral Diseases. J. Clin. Med. 2020, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Neiers, F.; Charles, J.; Heydel, J.; Muñoz-González, C.; Feron, G.; Canon, F. Oral enzymatic detoxification system: Insights obtained from proteome analysis to understand its potential impact on aroma metabolization. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5516–5547. [Google Scholar] [CrossRef] [PubMed]
- Canon, F.; Neiers, F.; Guichard, E. Saliva and flavor perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.; Vastardis, H.; Mermelekas, G.; Gerasimidi-Vazeou, A.; Zoidakis, J.; Vougas, K. Saliva proteomics analysis offers insights on type 1 diabetes pathology in a pediatric population. Front. Physiol. 2018, 9, 444. [Google Scholar] [CrossRef]
- Boehlke, C.; Zierau, O.; Hannig, C. Salivary amylase—The enzyme of unspecialized euryphagous animals. Arch. Oral Biol. 2015, 60, 1162–1176. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.; Mateo, S.; Tecles, F.; Hirtz, C.; Escribano, D.; Cerón, J. Changes Occurring on the Activity of Salivary Alpha-Amylase Proteoforms in Two Naturalistic Situations Using a Spectrophotometric Assay. Biology 2021, 10, 227. [Google Scholar] [CrossRef]
- Perry, G.H.; Dominy, N.J.; Claw, K.G.; Lee, A.S.; Fiegler, H.; Redon, R.; Werner, J.; Villanea, F.A.; Mountain, J.L.; Misra, R.; et al. Diet and the evolution of human amylase gene copy number variation. Nat. Genet. 2007, 39, 1256–1260. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; Costa, G.; Cordeiro, C.; Pinheiro, C.; Amado, F.; Lamy, E. Salivary proteome and glucose levels are related with sweet taste sensitivity in young adults. Food Nutr. Res. 2017, 61, 1389208. [Google Scholar] [CrossRef] [Green Version]
- Marquezin, M.C.S.; Pedroni-Pereira, A.; Araujo, D.S.; Rosar, J.V.; Barbosa, T.S.; Castelo, P.M. Descriptive analysis of the masticatory and salivary functions and gustatory sensitivity in healthy children. Acta Odontol. Scand. 2016, 74, 443–448. [Google Scholar] [CrossRef]
- Genovese, A.; Piombino, P.; Gambuti, A.; Moio, L. Simulation of retronasal aroma of white and red wine in a model mouth system. Investigating the influence of saliva on volatile compound concentrations. Food Chem. 2009, 114, 100–107. [Google Scholar] [CrossRef]
- Piombino, P.; Genovese, A.; Esposito, S.; Moio, L.; Cutolo, P.P.; Chambery, A.; Severino, V.; Moneta, E.; Smith, D.P.; Owens, S.M.; et al. Saliva from Obese Individuals Suppresses the Release of Aroma Compounds from Wine. PLoS ONE 2014, 9, e85611. [Google Scholar] [CrossRef] [Green Version]
- Denker, M.; Parat-Wilhelms, M.; Drichelt, G.; Paucke, J.; Luger, A.; Borcherding, K.; Hoffmann, W.; Steinhart, H. Investigation of the retronasal flavour release during the consumption of coffee with additions of milk constituents by ‘Oral Breath Sampling’. Food Chem. 2006, 98, 201–208. [Google Scholar] [CrossRef]
- Pagès-Hélary, S.; Andriot, I.; Guichard, E.; Canon, F. Retention effect of human saliva on aroma release and respective contribution of salivary mucin and α-amylase. Food Res. Int. 2014, 64, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Feron, G.; Brulé, M.; Canon, F. Understanding the release and metabolism of aroma compounds using micro-volume saliva samples by ex vivo approaches. Food Chem. 2018, 240, 275–285. [Google Scholar] [CrossRef]
- Ijichi, C.; Wakabayashi, H.; Sugiyama, S.; Ihara, Y.; Nogi, Y.; Nagashima, A.; Ihara, S.; Niimura, Y.; Shimizu, Y.; Kondo, K.; et al. Metabolism of Odorant Molecules in Human Nasal/Oral Cavity Affects the Odorant Perception. Chem. Senses 2019, 44, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Brulé, M.; Martin, C.; Feron, G.; Canon, F. Molecular mechanisms of aroma persistence: From noncovalent interactions between aroma compounds and oral mucosa to metabolization of aroma compounds by saliva and oral cells. Food Chem. 2022, 373, 131467. [Google Scholar] [CrossRef] [PubMed]
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of astringency: Structural alteration of the oral mucosal pellicle by dietary tannins and protective effect of bPRPs. Food Chem. 2018, 253, 79–87. [Google Scholar] [CrossRef]
- Monneuse, M.-O.; Rigal, N.; Frelut, M.-L.; Hladik, C.-M.; Simmen, B.; Pasquet, P. Taste acuity of obese adolescents and changes in food neophobia and food preferences during a weight reduction session. Appetite 2008, 50, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Antonello, V.; Antonello, I.C.F.; Santos, C.A.D.L. Sensibilidade gustativa ao sal, natriúria e pressão arterial em indivíduos normotensos. Rev. Assoc. Méd. Brasileira 2007, 53, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Vilela, A.; Matos, S.; Abraão, A.S.; Lemos, A.M.; Nunes, F.M. Sucrose Replacement by Sweeteners in Strawberry, Raspberry, and Cherry Jams: Effect on the Textural Characteristics and Sensorial Profile—A Chemometric Approach. J. Food Process. 2015, 2015, 749740. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of Different Processing Treatments on Almond (Prunus dulcis) Bioactive Compounds, Antioxidant Activities, Fatty Acids, and Sensorial Characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.; Cruz, I.; Oliveira, I.; Pinto, A.; Pinto, T. Sensory and Nutraceutical Properties of Infusions Prepared with Grape Pomace and Edible-Coated Dried–Minced Grapes. Coatings 2022, 12, 443. [Google Scholar] [CrossRef]
- ISO 8589. International Organization for Standardization (ISO)—Sensory Analysis—General Guidance for the Design of Test Rooms. 2007. Available online: https://www.iso.org/standard/36385.html (accessed on 20 May 2021).
- ISO 3591. International Organization for Standardization (ISO)—Sensory Analysis—Apparatus—Wine-Tasting Glass. 1977. Available online: https://www.iso.org/standard/9002.html (accessed on 20 May 2021).
- Abnova. Lipase Assay Kit, Catalog Number KA1654, Version: 03. S.d. Available online: https://www.abnova.com/products/products_detail.asp?catalog_id=KA1654 (accessed on 30 January 2021).
- Alencar, N.M.M.; Ribeiro, T.G.; Barone, B.; Barros, A.P.A.; Marques, A.T.B.; Behrens, J.H. Sensory profile and check-all-that-apply (CATA) as tools for evaluating and characterizing syrah wines aged with oak chips. Food Res. Int. 2018, 124, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. Eur. Food Res. Technol. 2015, 241, 157–171. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.-H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Wu, G.; Zhang, H.; Jin, Q.; Wang, X. Deep-fried flavor: Characteristics, formation mechanisms, and influencing factors. Crit. Rev. Food Sci. Nutr. 2020, 60, 1496–1514. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef]
- Sreerama, L.; Hedge, M.; Sladek, N. Identification of a class aldehyde dehydrogenase in human saliva and increased levels of this enzyme, glutathione S-transferases, and DT-diaphorase in the saliva of subjects who continually ingest large quantities of coffee or broccoli. Clin. Cancer Res. 1995, 1, 1153–1163. [Google Scholar]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J. PTC/PROP Tasting: Anatomy, Psychophysics, and Sex Effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef]
- Dziezak, J.D. Acids: Natural Acids and Acidulants. In Encyclopedia of Food and Health, 1st ed.; Elsevier Ltd.: London, UK, 2016; Volume 1. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Tasting—A Professional Handbook. In Food Science and Technology International Series; Elsevier Ltd.: London, UK, 2009. [Google Scholar]
- Pires, M.A.; Pastrana, L.M.; Fuciños, P.; Abreu, C.S.; Oliveira, S.M. Sensorial Perception of Astringency: Oral Mechanisms and Current Analysis Methods. Foods 2020, 9, 1124. [Google Scholar] [CrossRef]
- Kensinger, E.A. Remembering the Details: Effects of Emotion. Emot. Rev. 2009, 1, 99–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasser, S.M.; Castro, N.; Feretic, B. Alcohol sensory processing and its relevance for ingestion. Physiol. Behav. 2015, 148, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemon, C.H.; Wilson, D.M.; Brasser, S.M. Differential neural representation of oral ethanol by central taste-sensitive neurons in ethanol-preferring and genetically heterogeneous rats. J. Neurophysiol. 2011, 106, 3145–3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blizard, D.A. Sweet and Bitter Taste of Ethanol in C57BL/6J and DBA2/J Mouse Strains. Behav. Genet. 2007, 37, 146. [Google Scholar] [CrossRef] [PubMed]
- Scinska, A.; Koros, E.; Habrat, B.; Kukwa, A.; Kostowski, W.; Bienkowski, P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend. 2000, 60, 199–206. [Google Scholar] [CrossRef]
- Cretin, B.N.; Dubourdieu, D.; Marchal, A. Influence of ethanol content on sweetness and bitterness perception in dry wines. LWT 2018, 87, 61–66. [Google Scholar] [CrossRef]
- McRae, J.M.; Ziora, Z.M.; Kassara, S.; Cooper, M.A.; Smith, P.A. Ethanol Concentration Influences the Mechanisms of Wine Tannin Interactions with Poly(l-proline) in Model Wine. J. Agric. Food Chem. 2015, 63, 4345–4352. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Espínola-Espínola, V.; López-Solís, R. Wine pH Prevails over Buffering Capacity of Human Saliva. J. Agric. Food Chem. 2016, 64, 8154–8159. [Google Scholar] [CrossRef]
- Gammacurta, M.; Lytra, G.; Marchal, A.; Marchand, S.; Barbe, J.C.; Moine, V.; de Revel, G. Influence of lactic acid bacteria strains on ester concentrations in red wines: Specific impact on branched hydroxylated compounds. Food Chem. 2018, 239, 252–259. [Google Scholar] [CrossRef]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Tenuta, L.; Tabchoury, C.; Cury, J. Conceitos de pH, sistemas tampão e solubilidade: Aplicação na odontologia. In Bioquímica Oral; Artes Medicas Ltd (Edit): São Paulo, Brazil, 2017. [Google Scholar]
- Dysvik, A.; Liland, K.H.; Myhrer, K.S.; Westereng, B.; Rukke, E.-O.; de Rouck, G.; Wicklund, T. Pre-fermentation with lactic acid bacteria in sour beer production. J. Inst. Brew. 2019, 125, 342–356. [Google Scholar] [CrossRef]
- Palmer, J.J. How to Brew: Everything You Need to Know to Brew Great Beer Every Time, 4th ed.; Brewers Publications: Boulder, CO, USA, 2017; ISBN 10. [Google Scholar]
- Yamakoshi, Y. Dental and Oral Biology, Biochemistry. In Biomedical Sciences; Elsevier: London, UK, 2014; ISBN 9780128012383. [Google Scholar] [CrossRef]
- Alpers, D.H. Carbohydrates | Digestion, Absorption, and Metabolism. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2003; pp. 881–887. ISBN 9780122270550. [Google Scholar] [CrossRef]
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int. J. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morquecho-Campos, P.; Bikker, F.J.; Nazmi, K.; de Graaf, K.; Laine, M.L.; Boesveldt, S. Impact of food odors signaling specific taste qualities and macronutrient content on saliva secretion and composition. Appetite 2019, 143, 104399. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Vecchio, R.; Moio, L. Differences in Astringency Subqualities Evaluated by Consumers and Trained Assessors on Sangiovese Wine Using Check-All-That-Apply (CATA). Foods 2021, 10, 218. [Google Scholar] [CrossRef]
- Skoluda, N.; Dhrami, I.; Nater, U.M. Factors contributing to stability and instability in alpha-amylase activity in diluted saliva samples over time. Psychoneuroendocrinology 2020, 121, 104847. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the way of reporting alpha-Amylase values in saliva in different naturalistic situations: A pilot study. PLoS ONE 2017, 12, e0180100. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Aguilar, M.D.; Vialaret, J.; de Périère, D.D.; Escribano, D.; Lehmann, S.; Tecles, F.; Cerón, J.J.; Hirtz, C. Variation of human salivary alpha-amylase proteoforms in three stimulation models. Clin. Oral Investig. 2019, 24, 475–486. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Lu, Q.; Liu, R.; Tian, J.; Huang, Y. Study on interaction between hu-man salivary a-amylase and sorghum procyanidin tetramer: Binding characteristics and structural analysis. Int. J. Biol. Macromol. 2018, 118, 1136–1141. [Google Scholar] [CrossRef]
- Ramsey, I.; Dinu, V.; Linforth, R.; Yakubov, G.E.; Harding, S.E.; Yang, Q.; Ford, R.; Fisk, I. Understanding the lost functionality of ethanol in non-alcoholic beer using sensory evaluation, aroma release and molecular hydrodynamics. Sci. Rep. 2020, 10, 20855. [Google Scholar] [CrossRef]
- Wang, S.; Olarte Mantilla, S.M.; Smith, P.A.; Stokes, R.J.; Smyth, E.H. Tribology and QCM-D approaches provide mechanistic insights into red wine mouthfeel, astringency sub-qualities and the role of saliva. Food Hydrocoll. 2021, 120, 106918. [Google Scholar] [CrossRef]
- Hu, X.; Karthik, P.; Chen, J. Enhanced oral oil release and mouthfeel perception of starch emulsion gels. Food Res. Int. 2021, 144, 110356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Chen, J. The starch hydrolysis and aroma retention caused by salivary α-amylase during oral processing of food. Curr. Opin. Food Sci. 2022, 43, 237–245. [Google Scholar] [CrossRef]
- Abreu, T.; Perestrelo, R.; Bordiga, M.; Locatelli, M.; Coïsson, J.D.; Câmara, J. The Flavor Chemistry of Fortified Wines—A Comprehensive Approach. Foods 2021, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.; Ferreira, R.; Nunes, F.; Correia, E. Creation and Acceptability of a Fragrance with a Characteristic Tawny Port Wine-Like Aroma. Foods 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Chianeh, Y.R.; Manjunath, R.; Prabhu, K.; Fernandes, D.; Vidyasagar, M.; Kamath, A. Protein thiols and butryrylcholinestrase in saliva of oral cancer patients. Indian J. Clin. Biochem. 2014, 29, 238–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoytcheva, M.; Montero, G.; Zlatev, R.A.; Leon, J.; Gochev, V. Analytical Methods for Lipases Activity Determination: A Review. Curr. Anal. Chem. 2012, 8, 400–407. [Google Scholar] [CrossRef]



| Taste Descriptor | Representative Substance | Concentration (Aqueous Solutions), g/L | Concentration (Hydroalcoholic Solutions), g/L |
|---|---|---|---|
| Sweet | Sucrose | 10.0 | 5.0 |
| Salty | Sodium chloride | 1.0 | N.p. |
| Acid | Malic acid | 0.2 | N.p. |
| Citric acid | 0.2 | 0.2 | |
| Lactic acid | 0.2 | 0.5 | |
| Succinic acid | 0.5 | 0.8 | |
| Bitter/astringency | Tannin | 0.5 | 0.5 |
| Solutions/Beverages Before Contact with Saliva | The pH of the Solutions/Beverages after Contact with Saliva | |||||
|---|---|---|---|---|---|---|
| Aqueous solutions | Taste descriptor | Representative Substances | pH | pH (M ± SD) | t | p |
| Sour | Malic acid 1 | 3.25 | 3.37 ± 0.042 | 7.378 | <0.001 | |
| Succinic acid 1 | 3.30 | 3.54 ± 0.047 | 13.221 | <0.001 | ||
| Citric acid 1 | 3.19 | 3.39 ± 0.059 | 8.862 | <0.001 | ||
| Lactic acid 1 | 3.31 | 3.49 ± 0.052 | 8.984 | <0.001 | ||
| Bitter | Tannin 1 | 3.72 | 4.34 ± 0.349 | 4.732 | 0.003 | |
| Sweet | Sucrose 1 | 5.67 | 5.98 ± 0.175 | 4.727 | 0.003 | |
| Salty | Sodium chloride 1 | 4.96 | 5.92 ± 0.195 | 13.008 | <0.001 | |
| Hydroalcoholic Solutions | Sour | Succinic acid 1 | 3.36 | 3.51 ± 0.124 | 3.403 | 0.011 |
| Citric acid 1 | 3.36 | 3.53 ± 0.081 | 5.901 | 0.001 | ||
| Lactic acid 1 | 3.26 | 3.29 ± 0.047 | 1.504 | 0.176 | ||
| Bitter | Tannin 1 | 3.88 | 4.36 ± 0.107 | 12.716 | <0.001 | |
| Sweet | Sucrose 1 | 6.19 | 6.70 ± 0.189 | 11.630 | <0.001 | |
| Alcoholic beverages | Type of Beverage | Alcohol (%, v/v) | pH | pH (M ± SD) | t | p |
| Colorless brandy or Grappa (Bottle-aged) | 41.0 | 4.30 | 4.84 ± 0.249 | 6.824 | <0.001 | |
| Color brandy or Aguardente Velha (Wood-aged) | 40.0 | 3.93 | 4.45 ± 0.268 | 6.091 | <0.001 | |
| White wine | 13.0 | 3.37 | 3.22 ± 0.123 | −3.902 | 0.004 | |
| Red wine | 13.5 | 3.87 | 3.74 ± 0.119 | −3.400 | 0.008 | |
| Blonde beer | 4.9 | 4.34 | 4.34 ± 0.066 | 0.076 | 0.941 | |
| Succinic Acid M ± SD | Citric Acid M ± SD | Lactic Acid M ± SD | Malic Acid M ± SD | Sucrose M ± SD | Tannin M ± SD | Sodium Chloride M ± SD | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Aqueous | α-Amyl. mU/mL | 49.6 ± 36.8 b | 24.05 ± 31.5 b | 9.33 ± 15.1 b | 34.96 ± 56.4 b | 93.28 ± 22.5 c | 0.49 ± 0.86 a | 100.91 ± 20.8 c | 13.11 | 0.041 |
| Lipase U/L | -* | -* | -* | -* | -* | -* | -* | -* | -* | |
| Hydroalcoholic | α-Amyl. mU/mL | 44.37 ± 18.7 a | 63.89 ± 21.1 b | 75.79 ± 17.5 b | N.p. | 85.21 ± 13.4 b | 0.82 ± 1.3 a | N.p. | 17.89 | 0.001 |
| Lipase U/L | -* | 57.96 ± 46.0 | -* | N.p. | -* | -* | N.p. | -* | -* |
| Compounds | Aqueous M ± SD | Hydroalcoholic M ± SD | Z | p |
|---|---|---|---|---|
| Succinic acid | 49.67 ± 36.79 | 44.37 ± 18.68 | −0.149 | 0.881 |
| Citric acid | 24.05 ± 31.46 | 63.89 ± 21.09 | −1.640 | 0.101 |
| Lactic acid | 9.33 ± 15.12 | 75.79 ± 17.53 | −2.236 | 0.025 |
| Sucrose | 93.28 ± 22.47 | 85.21 ± 13.35 | −0.447 | 0.655 |
| Tannin | 0.49 ± 0.86 | 0.82 ± 1.29 | −0.764 | 0.445 |
| Colorless Brandy M ± SD | Color Brandy M ± SD | White Wine M ± SD | Red Wine M ± SD | Blonde Beer M ± SD | F | p | |
|---|---|---|---|---|---|---|---|
| α—Amylase mU/mL | 148.11 ± 31.15 c | 44.18 ± 38.68 ab | 4.15 ± 4.78 a | 13.84 ± 4.85 b | 2.03 ± 1.61 a | 56.59 | <0.001 |
| Lipase U/L | 41.49 ± 1.58 | 400.80 ± 135.88 | 270.65 ± 220.89 | -* | 63.84 ± 19.29 | 4.027 | 0.205 |
| Red Wine M ± SD | Port Wine M ± SD | p | ||
|---|---|---|---|---|
| α-Amylase (mU/mL) | 28.62 ± 49.54 | 72.55 ± 65.64 | −2.90 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.J.; Correia, E.; Vilela, A. Exploring the Impact of α-Amylase Enzyme Activity and pH on Flavor Perception of Alcoholic Drinks. Foods 2023, 12, 1018. https://doi.org/10.3390/foods12051018
Santos MJ, Correia E, Vilela A. Exploring the Impact of α-Amylase Enzyme Activity and pH on Flavor Perception of Alcoholic Drinks. Foods. 2023; 12(5):1018. https://doi.org/10.3390/foods12051018
Chicago/Turabian StyleSantos, Maria João, Elisete Correia, and Alice Vilela. 2023. "Exploring the Impact of α-Amylase Enzyme Activity and pH on Flavor Perception of Alcoholic Drinks" Foods 12, no. 5: 1018. https://doi.org/10.3390/foods12051018