Anthracnose Controlled by Essential Oils: Are Nanoemulsion-Based Films and Coatings a Viable and Efficient Technology for Tropical Fruit Preservation?
Abstract
:1. Introduction
2. Anthracnose in Tropical Fruits
2.1. Economic Data
2.2. The Infectious Process of Tropical Fruits by Colletotrichum
2.3. The Colletotrichum Lifestyle and Its Relationship with the Symptoms of Anthracnose
3. Conventional Treatments for Anthracnose Prevention
3.1. Physical Treatments
3.2. Chemical Treatments
4. Essential Oils
4.1. General Characteristics and Potential Applications
4.2. Mechanism of Action against Fungi
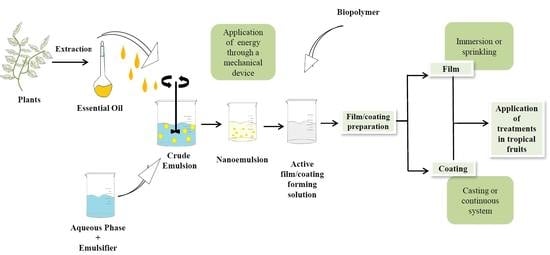
5. Active Films and Coatings
6. Emulsions
6.1. Conventional Emulsions
6.2. Nanoemulsions
6.3. Advantages of Using Nanoemulsions over Conventional Methods
7. Current Scenario: Essential Oils in Active Film and Coating Formulations
| Essential Oil | Application of Essential Oil | Coating/Film | Species | Fruits | Main Results | Author |
|---|---|---|---|---|---|---|
| Copaiba (Copaifera langsdorffii), cloves (Syzygium aromaticum), eucalyptus (Eucalyptus citriodora), and rosemary (Rosmarinus officinalis) | Direct under the isolated strains | - | C. musae; C. gloeosporioides | Mango and banana | Inhibition of mycelial growth in vitro | [60] |
| Mint (Mentha piperita L. and Mentha × villosa Huds) | Emulsion | Chitosan coating | C. gloeosporioides; C. brevisporum | Papaya | Total inhibition of mycelial growth in vitro; total inhibition of lesion development in vivo | [81] |
| Garlic (Allium sativum), copaiba (Copaifera langsdorfii), clove (Eugenia caryophyllata), and cinnamon (Cinnamomum zeylanicum) | Emulsion | - | C. musae | Banana | Better results of biomass loss, fruit diameter, and length; lower incidence and severity of the disease | [80] |
| Thyme (Thymus vulgaris L.) | Direct application to the coating | Chitosan, gum arabic, and Aloe vera coatings | C. gloeosporioides | Avocado | Increased activity of antioxidant enzymes and consequent delay in cell degeneration caused by C. gloeosporioides; delay in the spread of the disease | [107] |
| Thyme (species not identified by the author) | Direct application to the coating | Chitosan coating | C. gloeosporioides | Avocado | The treated fruits were firmer and had a lower incidence of C. gloeosporioides | [108] |
| Mint (Mentha piperita L.) | Emulsion | Chitosan coating | C. asianum, C. fructicola, C. tropicale, C. dianesei, and C. karstii | Mango | Decreased mycelial growth and decreased severity of lesion to fruit | [109] |
| Clove (Eugenia caryophyllata), lemongrass (Cymbopogon citratus), thyme (Thymus vulgaris), cinnamon (Cinnamomum zeylanicum), and oregano (Origanum vulgare) | Direct application to the film | Cassava starch film | C. musae | Banana | Inhibition of mycelial growth and in vitro germination; reduction in and prevention of anthracnose lesions in fruits | [82] |
| Clove (Caryophillus aromaticus L.), almond (Acrocomia aculeata), and neem (Azadiractha indica A.Juss) | Spraying on fruits | - | C. gloeosporioides | Mango | Lower rates of fruit mass loss and lower incidence and progression of anthracnose | [110] |
| Lemongrass (species not shown) and cinnamon (Cinnamomum zeylanicum) | Direct application to the film | Gum arabic coating | C. musae and C. gloeosporioides | Banana and papaya | 80% anthracnose control in bananas and 71% in papaya | [111] |
| Oregano (Origanum vulgaris) and lemongrass (Cymbopogon | Incorporated in sachets | - | C. gloeosporides | Mango | Control of fungal development in vitro | [112] |
| Lemongrass (Cymbopogon citratus), cinnamon (Cinnamomum zeylanicum), anise (Pimpinella anisum), rosemary (Rosmarinus officinalis), tea tree (Melaleuca alternifolia), and mint (Mentha piperita) | Direct application to the fruits | - | C. gloeosporioides | Papaya | Fungitoxic (in vitro) and fungistatic (in vivo) effect and lesion reduction | [8] |
| Clove (Syzygium aromaticum) | Direct application to the coating | Cassava starch coating | C. gloeosporioides | Guava | Increased shelf life, delayed color change, and slowed down the evolution of anthracnose | [113] |
| Lemongrass (Cymbopogon citratus) | Emulsion | Chitosan coating | C. asianum; C. siamense; C. fructicola; C. karstii; C.tropicale | Guava, mango, and papaya | Inhibition of fungal growth in vitro; partial and total inhibition of lesion development in fruits | [2] |
| Basil (Ocimum basilicum) | Emulsion | Beeswax coating | C. gloeosporioides | Mango | Control of anthracnose in fruits and absence of physicochemical alterations | [114] |
| Noni (Morinda citrifolia L.), lemongrass (Cymbopogon citratus DC Stapf), and mastruz (Chenopodium ambrosioides) | Direct application to the coating | Carnauba wax, gelatin, paraffin, and sunflower oil coatings | C. gloeosporioides | Papaya | Reduction in fungal growth rate and inhibition of conidia germination; reduction of at least 7% in fruit mass loss | [115] |
| Thymol and carvacrol | Direct application to the film | Starch film | C. gloeosporioides | Papaya and mango | Reduced incidence of anthracnose symptoms in treated fruits; in vitro fungicidal and in vivo fungistatic effects | [116] |
| Thyme (Thymus vulgaris L.) | Direct application to the fruits | - | C. musae | Banana | Delay in fruit ripening and increased shelf life; reduction of 37.2% of disease in fruits treated with the essential oil | [1] |
| Rue (Ruta graveolens L.) | Emulsion | Chitosan coating | C. gloesporoides | Papaya | Significant inhibition of Colletotrichum gloeosporioides growth in vitro and in situ; the organoleptic characteristics of the fruits were not affected | [117] |
| Cinnamon (Cinnamomum cassia) | Emulsion | Chitosan, sodium alginate, and carrageenan coating | C. acutatum | Strawberries | No significant differences between the control and treated samples in sensory evaluation | [118] |
| Thyme (Thymus daenensis Celak), lavender (Lavandula angustifolia Mill.), mint (Mentha piperita Willd.), savory (Satureja khuzistanica Jamzad.), and cinnamon (Cinnamomum zeylanicum Blume) | Spraying on fruits | - | C. gloesporoides | Papaya | Improved fruit firmness and reduced lesion diameter; reduced levels of fruit decomposition | [119] |
| Thyme (Thymus vulgaris) and Mexican lime (Citrus aurantifolia) | Emulsion | Mesquite gum candelilla wax and mineral oil coating | C. gloeosporioides | Papaya | Control of fungal growth; reduction in infection and disease severity indices | [120] |
| Lemon ironbark (Eucalyptus staigeriana), pepper rosemary (Lippia sidoides), and louro-cravo (Pimenta pseudocaryophyllus) | Emulsion | Carboxymethylcellulose coating | C. acutatum | Strawberries | Good fungal inhibition capacity (in vitro); morphological degradation of hyphae; reduced severity of anthracosis in fruits | [121] |
| Ginger (species not identified by the author) | Direct application to the coating | Gum arabic coating | C. gloeosporioides | Papaya | Maintenance of firmness, color, acid titration, and fruit ripening; inhibition of fungal development. | [122] |
| Thyme (Thymus vulgaris L.) | Emulsion | Chitosan coating | C. gloeosporioides | Mango | Inhibition of fungal growth in vitro; control of disease symptoms in vivo | [123] |
| Thymol and R-(−)-carvone | Direct application to the film | Poly(lactic acid) (PLA)-based polymer film | C. gloeosporioides | Avocado | Suppression of mycelial growth in vivo | [124] |
| Common rue (Ruta graveolens) | Emulsion | Chitosan coating | C. gloeosporioides | Guava | Reduced fungal growth and increased stability for at least 12 days; promising results for mass loss, maturation index, respiratory rate, color, firmness, and water activity; inhibition of lesion development | [125] |
| Cinnamon (Cinnamomum zeylanicum or Cinnamomum verum) | Direct under the isolated strains | - | C. acutatum | Kiwifruit | Reduction in fungal growth and spore germination | [9] |
| Yarrow (Achillea millefolium), Ferula kuma, and mint (Mentha longifolia) | Direct application to the fruits | - | C. nymphaeae | Strawberry | Reduction in fruit rot caused by the fungus | [126] |
| Thyme (Thymus daenensis Celak.), savory (Satureja khuzistanica Jamzad.), mint (Mentha piperita Willd.), cinnamon (Cinnamomum zeylanicum Blume.), and lavender (Lavandula angustifolia Mill.) | Emulsion sprayed on the fruits | - | C. gleoporiodes | Avocado | Reduction in disease severity | [127] |
| Lemon-scented ironbark (Eucalyptus staigeriana), rosemary pepper (Lippia sidoides), and louro-cravo (Pimenta pseudocaryophyllu) | Direct application to the coating | Carboxymethylcellulose coating | C. gloeosporioides | Papaya | Reduction in disease severity | [128] |
| Basil (Ocimum basilicum and Ocimum gratissimum) | Emulsion | - | C. musae | Banana | Inhibition of growth in situ and in vivo | [129] |
| Basil (Ocimum basilicum), cinnamon (Cinnamomum zeylanicum), and rosemary (Rosmarinus officinalis) | Spraying on fruits | - | C. musae | Banana | Reduction in mycelial growth in vitro; decreased incidence of anthracnose in fruits and increased shelf life | [130] |
| Thyme (Thymus vulgaris), cinnamon bark (Cinnamomum zeylanicum Blume), and clove bud (Syzygium aromaticum) | Direct application to the fruits | - | C. acutatum | Strawberry | Decreased development of the disease | [131] |
| Lemongrass (Cymbopogon citratus L.) | Nanocapsules constituted by essential oil and polylactic acid | - | C. acutatum and C. gloeosporioides | Apples | Reduction in rot lesions | [132] |
| Garlic (Allium sativum L.) | Direct application to the coating | Aloe vera coating | C. musae | Banana | Increased resistance against disease development | [133] |
| Pepper tree (Schinus molle) | Essential oil nanoparticles | Chitosan nanoparticles coating | C. gloeosporioides | Avocado | Efficiency against the establishment of the fungus in fruits | [134] |
| Ginger, plai, and fingerroot (species not identified by the author) | Emulsion | Hydroxypropyl methylcellulose coating | C.gloeosporioides | Mango | Inhibition of fungal growth and development | [135] |
| Tea tree (species not identified by the author) | Nanoemulsion | Sodium alginate film | C. musae | Banana | Inhibition of fungal growth and reduction in anthracnose symptoms in fruits | [100] |
| Black mustard (Brassica nigra) | Nanoemulsion | - | C. musae and C. capsici | - | Low inhibition of mycelial growth | [136] |
| D-Limonene | Nanoemulsion | - | C. gloeosporioides | - | Inhibition of mycelial growth | [137] |
| Cinnamon (Cinnamomum zeylanicum) | Nanoemulsion | - | C. gloeosporioides | - | Inhibition of fungal growth | [90] |
| Black pepper (P. nigrum L.), cinnamon (C. zeylanicum Blume), Jamaica pepper (P. dioica Lindl.), clove (S. aromaticum (L.) Merr), anise (Illicium verum Hook. f.), and nutmeg (Myristica fragrans Houtt) | Nanoemulsion | - | C. gloeosporioides | - | Inhibition of fungal growth | [138] |
| Ginger oil (species not identified by the author) | Nanoemulsion | Carnauba wax coating | C. gloeosporioides | Papaya | Inhibition of the mycelial zone and effective control upon fungal growth in fruits | [102] |
| Clove (Syzygium aromaticum) | Nanoemulsion | - | C. gloeosporioides | - | Inhibition of fungal growth | [139] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilaplana, R.; Pazmiño, L.; Valencia-Chamorro, S. Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol. Technol. 2018, 138, 56–63. [Google Scholar] [CrossRef]
- Lima Oliveira, P.D.; de Oliveira, K.Á.R.; Vieira, W.A.D.S.; Câmara, M.P.S.; de Souza, E.L. Control of anthracnose caused by Colletotrichum species in guava, mango and papaya using synergistic combinations of chitosan and Cymbopogon citratus (D.C. ex Nees) Stapf. essential oil. Int. J. Food Microbiol. 2018, 266, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Gasca, C.A.; Dassoler, M.; Dotto Brand, G.; de Medeiros Nóbrega, Y.K.; Gomes, S.M.; Masrouah Jamal, C.; de Oliveira Magalhães, P.; Fonseca-Bazzo, Y.M.; Silveira, D. Chemical composition and antifungal effect of ethanol extract from Sapindus saponaria L. fruit against banana anthracnose. Sci. Hortic. 2020, 259, 108842. [Google Scholar] [CrossRef]
- Shivaprakash, M.R.; Appannanavar, S.B.; Dhaliwal, M.; Gupta, A.; Gupta, S.; Gupta, A.; Chakrabarti, A. Colletotrichum truncatum: An unusual pathogen causing mycotic keratitis and endophthalmitis. J. Clin. Microbiol. 2011, 49, 2894–2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paniz-Mondolfi, A.E.; Agemy, S.; Cañete-Gibas, C.; Gitman, M.R.; Iacob, C.E.; Necula, I.; Nowak, M.D. First report of human infection caused by Colletotrichum chlorophyti occurring in a post-corneal transplant patient with endophthalmitis. Med. Mycol. Case Rep. 2021, 32, 73–76. [Google Scholar] [CrossRef]
- van der Ven, L.T.M.; Rorije, E.; Sprong, R.C.; Zink, D.; Derr, R.; Hendriks, G.; Loo, L.H.; Luijten, M. A Case Study with Triazole Fungicides to Explore Practical Application of Next-Generation Hazard Assessment Methods for Human Health. Chem. Res. Toxicol. 2021, 33, 834–848. [Google Scholar] [CrossRef]
- Mesías, F.J.; Martín, A.; Hernández, A. Consumers’ growing appetite for natural foods: Perceptions towards the use of natural preservatives in fresh fruit. Food Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef]
- Andrade, W.P.; Vieira, G.H.C. Efeito dos óleos essenciais sobre a antracnose in vitro e em frutos de mamoeiro. Rev. Bras. Plantas Med. 2016, 18, 367–372. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Wu, D.; Zhang, Q.; Chen, H.; Li, H.; Han, Q.; Lai, X.; Wang, H.; Wu, Y.; Yuan, J.; et al. Efficacy and mechanism of cinnamon essential oil on inhibition of Colletotrichum acutatum isolated from “Hongyang” kiwifruit. Front. Microbiol. 2018, 9, 1288. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; de Melo, N.R.; Andrade, M.; Sanches-Silva, A. Potential of migration of active compounds from protein-based films with essential oils to a food and a food simulant. Packag. Technol. Sci. 2017, 30, 791–798. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef] [Green Version]
- Lago, A.M.T.; Neves, I.C.O.; Oliveira, N.L.; Botrel, D.A.; Minim, L.A.; de Resende, J.V. Ultrasound-assisted oil-in-water nanoemulsion produced from Pereskia aculeata Miller mucilage. Ultrason. Sonochem. 2019, 50, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.D.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- de Capdeville, G.; Souza, M.T.; Santos, J.R.P.; de Paula Miranda, S.; Caetano, A.R.; Torres, F.A.G. Selection and testing of epiphytic yeasts to control anthacnose in post-harvest of papaya fruit. Sci. Hortic. 2007, 111, 179–185. [Google Scholar] [CrossRef]
- Bahia, S.R.N. Companhia Nacional de Abastecimento (Conab). Bol. Hortigranj. 2021, 7. Available online: https://www.conab.gov.br/info-agro/hortigranjeiros-prohort/boletim-hortigranjeiro (accessed on 19 April 2021).
- FAO. Redução de Perdas e Desperdícios Alimentares é Essencial Para Alcançar Metas Globais. 2019. Available online: http://www.fao.org/brasil/noticias/detail-events/pt/c/1199506/ (accessed on 14 October 2019).
- Udayanga, D.; Manamgoda, D.S.; Liu, X.; Chukeatirote, E.; Hyde, K.D. What are the common anthracnose pathogens of tropical fruits? Fungal Divers. 2013, 61, 165–179. [Google Scholar] [CrossRef]
- Botelho, L.N.S.; Rocha, D.A.; Braga, M.A.; Silva, A.; de Abreu, C.M.P. Quality of guava cv. ‘Pedro Sato’ treated with cassava starch and cinnamon essential oil. Sci. Hortic. 2016, 209, 214–220. [Google Scholar] [CrossRef]
- Perkins, M.L.; Joyce, D.C.; Coates, L.M. Possible contribution of impact injury at harvest to anthracnose expression in ripening avocado: A review. Sci. Hortic. 2019, 246, 785–790. [Google Scholar] [CrossRef]
- Stergiopoulos, L.; Gordon, T.R. Cryptic fungal infections: The hidden agenda of plant pathogens. Front. Plant Sci. 2014, 5, 506. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and necrotrophic lifestyle choice during postharvest disease development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.D.; Crous, P.W.; Ades, P.K.; Hyde, K.D.; Taylor, P.W.J. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017, 31, 155–168. [Google Scholar] [CrossRef]
- Calo, L.; García, I.; Gotor, C.; Romero, L.C. Leaf hairs influence phytopathogenic fungus infection and confer an increased resistance when expressing a Trichoderma α-1,3-glucanase. J. Exp. Bot. 2006, 57, 3911–3920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J.P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 274. [Google Scholar] [CrossRef] [Green Version]
- Wharton, P.S.; Schilder, A.C. Novel infection strategies of Colletotrichum acutatum on ripe blueberry fruit. Plant Pathol. 2008, 57, 122–134. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, V.P.; Babu, A.M.; Mishra, R.K.; Thiagarajan, V.; Datta, R.K. Surface ultrastructural studies on penetration and infection process of Colletotrichum gloeosporioides on mulberry leaf causing black spot disease. J. Phytopathol. 2001, 149, 629–633. [Google Scholar] [CrossRef]
- Kodama, S.; Nishiuchi, T.; Kubo, Y. Colletotrichum orbiculare MTF4 is a key transcription factor downstream of mor essential for plant signal-dependent appressorium development and pathogenesis. Mol. Plant-Microbe Interact. 2019, 32, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, F.T.; Moreno, J.; García-Herdugo, G.; De Los Santos, B.; Barrau, C.; Porras, M.; Blanco, C.; Romero, F. Ultrastructure of the early stages of Colletotrichum acutatum infection of strawberry tissues. Can. J. Bot. 2005, 83, 491–500. [Google Scholar] [CrossRef]
- Moraes, S.R.G.; Escanferla, M.E.; Massola, N.S. Prepenetration and penetration of Colletotrichum gloeosporioides into guava fruit (Psidium guajava L.): Effects of temperature, wetness period and fruit age. J. Phytopathol. 2015, 163, 149–159. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Afroz, T.; Lee, Y.G.; Kim, B.S. Post-harvest anthracnose of papaya caused by Colletotrichum truncatum in Korea. Eur. J. Plant Pathol. 2018, 150, 259–265. [Google Scholar] [CrossRef]
- Korsten, L. Advances in control of postharvest diseases in tropical fresh produce. Int. J. Postharvest Technol. Innov. 2006, 1, 48–61. [Google Scholar] [CrossRef]
- Alvindia, D.G.; Acda, M.A. Revisiting the efficacy of hot water treatment in managing anthracnose and stem-end rot diseases of mango cv. “Carabao”. Crop Prot. 2015, 67, 96–101. [Google Scholar] [CrossRef]
- Fischer, I.H.; de Arruda Palharini, M.C.; Jeronimo, E.M.; Sartori, M.M.P.; Sanhueza, R.M.V. Eficiência de radiação UV-C no controle da antracnose e da podridão peduncular em abacate ‘Hass’. Agropecuária Científica No Semiárido 2019, 15, 10. [Google Scholar] [CrossRef] [Green Version]
- Wanasinghe, W.U.T.; Damunupola, J.W. Effect of UV-C hormesis in regulating anthracnose disease and postharvest quality of tomato. J. Agric. Sci.—Sri Lanka 2020, 15, 318–327. [Google Scholar] [CrossRef]
- Rocha, A.F.; Ferreira, N.N.; Souza, A.R.M.; Flores, I.J.; Arthur, V. Aceitação e consumo de alimentos irradiados em Goiânia-GO. Braz. J. Anim. Environ. Res. 2021, 4, 1618–1632. [Google Scholar] [CrossRef]
- Vieira, W.A.D.S.; Lima, W.G.; Nascimento, E.S.; Michereff, S.J.; Reis, A.; Doyle, V.P.; Câmara, M.P.S. Thiophanate-methyl resistance and fitness components of Colletotrichum musae isolates from banana in Brazil. Plant Dis. 2017, 101, 1659–1665. [Google Scholar] [CrossRef] [Green Version]
- Tavares, G.M.; de Souza, P.E. Efeito de fungicidas no controle in vitro de Colletotrichum gloeosporioides, agente etiológico da antracnose do mamoeiro (Carica papaya L.). Ciência Agrotecnologia 2005, 29, 52–59. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, J.; Shin, S.-C.; Lee, S.-G.; Park, I.-K. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 23–28. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Dima, S. Essential oils in foods: Extraction, stabilization, and toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; el Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; de Martino, L.; Coppola, R.; de Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involve in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2019, 78, 61–71. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Chavan, P.S.; Tupe, S.G. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control 2014, 46, 115–120. [Google Scholar] [CrossRef]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; de Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Rasooli, I.; Rezaei, M.B.; Allameh, A. Growth inhibition and morphological alterations of Aspergillus niger by essential oils from Thymus eriocalyx and Thymus x-porlock. Food Control 2006, 17, 359–364. [Google Scholar] [CrossRef]
- Ahmadi, R.; Kalbasi-Ashtari, A.; Oromiehie, A.; Yarmand, M.S.; Jahandideh, F. Development and characterization of a novel biodegradable edible film obtained from psyllium seed (Plantago ovata Forsk). J. Food Eng. 2012, 109, 745–751. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skandamis, P.N.; Nychas, G.J.E. Preservation of fresh meat with active and modified atmosphere packaging conditions. Int. J. Food Microbiol. 2002, 79, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Fong, W.P.; Tsang, P.W.K. Novel antifungal activity of purpurin against Candida species in vitro. Med. Mycol. 2010, 48, 904–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Pereira, M.C.; Vilela, G.R.; Costa, L.M.A.S.; Silva, R.F.D.; Fernandes, A.F.; Fonseca, E.W.N.D.; Piccoli, R.H. Inhibition fungi growth through of utilization essential oils of spice. Ciência Agrotecnologia 2006, 30, 731–738. [Google Scholar] [CrossRef] [Green Version]
- De Araújo, A.C.; Toledo, E.D.; de Oliveira Soares, W.R. Produtos alternativos no controle de Colletotrichum spp. isolados de manga e banana. Científic@—Multidiscip. J. 2018, 5, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Venturoso, L.R.; Bacchi, L.M.A.; Gavassoni, W.L.; Conus, L.A.; Pontim, B.C.A.; Bergamin, A.C. Atividade antifúngica de extratos vegetais sobre o desenvolvimento de fitopatógenos. Summa Phytopathol. 2011, 37, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible films for food packaging: A review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Mkandawire, M.; Aryee, A.N. Resurfacing and modernization of edible packaging material technology. Curr. Opin. Food Sci. 2018, 19, 104–112. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2019, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effectiveness of alginate coating on antioxidant enzymes and biochemical changes during storage of mango fruit. J. Food Biochem. 2019, 43, e12990. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N.E.; Rayan, A.M. Effect of different edible coatings on biochemical quality and shelf life of apricots (Prunus armenica L. cv Canino). J. Food Meas. Charact. 2019, 13, 3173–3182. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, M.A. Modified atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Galus, S.; Kibar, E.A.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Han, J.H. Edible Films and Coatings: A Review. In Innovations in Food Packaging, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 213–255. [Google Scholar]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.W.; Cao, J.; Jiang, W. Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 2022, 367, 130671. [Google Scholar] [CrossRef] [PubMed]
- Pola, C.C.; Medeiros, E.A.; Pereira, O.L.; Souza, V.G.; Otoni, C.G.; Camilloto, G.P.; Soares, N.F. Cellulose acetate active films incorporated with oregano (Origanum vulgare) essential oil and organophilic montmorillonite clay control the growth of phytopathogenic fungi. Food Packag. Shelf Life 2016, 9, 69–78. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Affes, S.; Boufi, S.; Nasri, M. Optimization of the formulation of chitosan edible coatings supplemented with carotenoproteins and their use for extending strawberries postharvest life. Food Hydrocoll. 2018, 83, 375–392. [Google Scholar] [CrossRef]
- de Figueiredo Sousa, H.A.; de Oliveira Filho, J.G.; Egea, M.B.; da Silva, E.R.; Macagnan, D.; Pires, M.; Peixoto, J. Active film incorporated with clove essential oil on storage of banana varieties. Nutr. Food Sci. 2019, 49, 911–924. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Serdaroğlu, M.; Öztürk, B.; Kara, A. An Overview of Food Emulsions: Description, Classification and Recent Potential Applications. Turk. J. Agric.—Food Sci. Technol. 2015, 3, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Cruz, M.E.S.; Schwan-Estrada, K.R.F.; Clemente, E.; Itako, A.T.; Stangarlin, J.R.; Cruz, M.J.S. Plant extracts for controlling the post-harvest anthracnose of banana fruit. Rev. Bras. Plantas Med. 2013, 15, 727–733. [Google Scholar] [CrossRef] [Green Version]
- dos Passos Braga, S.; Lundgren, G.A.; Macedo, S.A.; Tavares, J.F.; dos Santos Vieira, W.A.; Câmara, M.P.S.; de Souza, E.L. Application of coatings formed by chitosan and Mentha essential oils to control anthracnose caused by Colletotrichum gloesporioides and C. brevisporum in papaya (Carica papaya L.) fruit. Int. J. Biol. Macromol. 2019, 139, 631–639. [Google Scholar] [CrossRef]
- de Oliveira Soares, M.G.; Alves, E.; de Freitas, A.S.; de Almeida, C.A.; da Silva Costa, S. Control of anthracnose in banana with cassava starch film associated or not with essential oils. Rev. Bras. Cienc. Agrar. 2020, 15, e8258. [Google Scholar]
- Jin, W.; Xu, W.; Liang, H.; Li, Y.; Liu, S.; Li, B. Nanoemulsions for food: Properties, production, characterization, and applications. Emulsions 2016, 3, 1–36. [Google Scholar]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Salem, M.A.; Ezzat, S.M. Nanoemulsions in food industry. In Some New Aspects of Colloidal Systems in Foods; IntechOpen: London, UK, 2019. [Google Scholar]
- Espitia, P.J.P.; Fuenmayor, C.A.; Otoni, C.G. Nanoemulsions: Synthesis, characterisation, and application in bio-based active food packaging. Compr. Rev. Food Sci. Food Saf. 2018, 18, 264–281. [Google Scholar] [CrossRef]
- Komaiko, J.; McClements, D.J. Food-grade nanoemulsion filled hydrogels formed by spontaneous emulsification and gelation: Optical properties, rheology, and stability. Food Hydrocoll. 2015, 46, 67–75. [Google Scholar] [CrossRef]
- Alliod, O.; Valour, J.P.; Urbaniak, S.; Fessi, H.; Dupin, D.; Charcosset, C. Preparation of oil-in-water nanoemulsions at large-scale using premix membrane emulsification and Shirasu porous glass (SPG) membranes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 76–84. [Google Scholar] [CrossRef]
- Anwer, M.K.; Jamil, S.; Ibnouf, E.O.; Shakeel, F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J. Oleo Sci. 2014, 63, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Pongsumpun, P.; Iwamoto, S.; Siripatrawan, U. Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrason. Sonochem. 2020, 60, 104604. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, structure, and physical properties. J. Phys. Condens. Matter 2006, 19, 079001. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Impact of microfluidization or ultrasound processing on the antimicrobial activity against Escherichia coli of lemongrass oil-loaded nanoemulsions. Food Control 2014, 37, 292–297. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Yadav, A.; Kumar, A.; Singh, P.P.; Dubey, N.K. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control 2018, 89, 1–11. [Google Scholar] [CrossRef]
- Sharma, S.; Cheng, S.F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.P.; Srivastava, P. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Adv. Colloid Interface Sci. 2021, 287, 102318. [Google Scholar]
- Ghadetaj, A.; Almasi, H.; Mehryar, L. Development and characterization of whey protein isolate active films containing nanoemulsions of Grammosciadium ptrocarpum Bioss. essential oil. Food Packag. Shelf Life 2018, 16, 31–40. [Google Scholar] [CrossRef]
- Abdollahzadeh, E.; Nematollahi, A.; Hosseini, H. Composition of antimicrobial edible films and methods for assessing their antimicrobial activity: A review. Trends Food Sci. Technol. 2021, 110, 291–303. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, T.; Gao, C.; Liu, X.; Zhang, N.I.; Feng, X.; Tang, X. Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int. J. Biol. Macromol. 2019, 122, 388–394. [Google Scholar] [CrossRef]
- Won, J.S.; Lee, S.J.; Park, H.H.; Song, K.B.; Min, S.C. Edible Coating Using a Chitosan-Based Colloid Incorporating Grapefruit Seed Extract for Cherry Tomato Safety and Preservation. J. Food Sci. 2018, 83, 138–146. [Google Scholar] [CrossRef]
- Yang, R.; Miao, J.; Shen, Y.; Cai, N.; Wan, C.; Zou, L.; Chen, C.; Chen, J. Antifungal effect of cinnamaldehyde, eugenol and carvacrol nanoemulsion against Penicillium digitatum and application in postharvest preservation of citrus fruit. LWT 2021, 141, 110924. [Google Scholar] [CrossRef]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef]
- Miranda, M.; Sun, X.; Assis, O.B.G.; Ference, C.; Ferreira, M.D.; Baldwin, E.A. Antifungal activity of Zingiber officinale Roscoe (ginger) oil and extracts, associated with carnauba wax nanoemulsions, on fungal control of harvest papaya. In ISHS Acta Horticulturae 1325, Proceedings of the V International Symposium on Postharvest Pathology: Consumer to Laboratory-Sustainable Approaches to Managing Postharvest, Liège, Belgium, 19–24 May 2019; ISHS: Leuven, Belgium, 2021; pp. 191–198. [Google Scholar]
- Yang, Z.; Li, M.; Zhai, X.; Zhao, L.; Elrashei Tahir, H.E.; Shi, J.; Zou, X.; Huanga, X.; Li, Z.; Xiao, J. Development and characterization of sodium alginate/tea tree essential oil nanoemulsion active film containing TiO2 nanoparticles for banana packaging. Int. J. Biol. Macromol. 2012, 213, 145–154. [Google Scholar] [CrossRef]
- Guilherme, E.O.; Costa, P.F.; de Souza Carlos Mourão, D.; Osorio, P.R.A.; de Souza Ferreira, T.P.; da Silva Oliveira, G.R.A.; Santos, G.R. Óleos Essenciais de plantas medicinais associados a biofilmes para proteção de frutos de mamoeiro. In Avanços Em Ciência Tecnologia Alimentos; Cardoso, R., Quintela, J.B., Oliveira, R.J., Eds.; Científica Digital: São Paulo, Brazil, 2021; pp. 305–320. [Google Scholar]
- Reis, J.B.; Figueiredo, L.A.; Castorani, G.M.; Veiga, S.M.O.M. Avaliação da atividade antimicrobiana dos óleos essenciais contra patógenos alimentares. Braz. J. Health Rev. 2020, 3, 342–363. [Google Scholar] [CrossRef]
- Chen, G.; Liu, B. Cellulose sulfate based film with slow-release antimicrobial properties prepared by incorporation of mustard essential oil and β-cyclodextrin. Food Hydrocoll. 2016, 55, 100–107. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina M, Á.; Hernández-López, M. The Effect of Nanostructured Chitosan and Chitosan-thyme Essential Oil Coatings on Colletotrichum gloeosporioides Growth in vitro and on cv Hass Avocado and Fruit Quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- de Oliveira, K.A.R.; Ramos Berger, L.R.; de Araújo, S.A.; Saraiva Câmara, M.P.; de Souza, E.L. Synergistic mixtures of chitosan and Mentha piperita L. essential oil to inhibit Colletotrichum species and anthracnose development in mango cultivar Tommy Atkins. Food Microbiol. 2017, 66, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Lemos, L.M.C.; Coutinho, P.H.; Salomão, L.C.C.; Siqueira, D.L.D.; Cecon, P.R. Controle da antracnose na pós-colheita de manga’Ubá’com o uso de produtos alternativos. Rev. Bras. Frutic. 2013, 35, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, M.; Ali, A.; Aldersona, P.G.; Mohamed, M.T.M.; Siddiqui, Y.; Zahid, N. Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol. Technol. 2011, 62, 71–76. [Google Scholar] [CrossRef]
- Medeiros, E.A.A.; Soares, N.D.F.F.; Polito, T.D.O.S.; Sousa, M.M.D.; Silva, D.F.P. Sachês antimicrobianos em pós-colheita de manga. Rev. Bras. Frutic. 2011, 33, 363–370. [Google Scholar] [CrossRef]
- De Souza Coelho, C.C.; de Oliveira Fonseca, M.J.; Soares, A.G.; Campos, R.D.S.; Freitas-Silva, O. Aplicação De Revestimento Filmogênico À Base De Amido De Mandioca E De Óleo De Cravo-Da-Índia Na Conservação Pós-Colheita De Goiaba ‘Pedro Sato’. Rev. Eng. Na Agric. 2017, 25, 479–490. [Google Scholar]
- Karunanayake, K.O.L.C.; Liyanage, K.C.M.; Jayakody, L.K.R.R.; Somaratne, S. Basil oil incorporated beeswax coating to increase shelf life and reduce anthracnose development in mango cv. Willard. Ceylon J. Sci. 2014, 49, 355–361. [Google Scholar] [CrossRef]
- Dias, B.L.; Costa, P.F.; Dakin, M.S.; Dias, F.R.; de Sousa, R.R.; de Souza Ferreira, T.P.; rigues Dos Santos, G.R. Control of papaya fruits anthracnose by essential oils of medicinal plants associated to different coatings. J. Med. Plants Res. 2020, 14, 239–246. [Google Scholar]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Avila-Sosa, R. Starch edible films/coatings added with carvacrol and thymol: In vitro and in vivo evaluation against Colletotrichum gloeosporioides. Foods 2020, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Ruiz, Y.; Grande Tovar, C.; Sinning-Mangonez, A.; Bermont, D.; P´erez Cordero, A.; Paparella, A.; Chaves-Lopez, C. Colletotrichum gloesporioides inhibition using chitosan-Ruta graveolens L essential oil coatings: Studies in vitro and in situ on Carica papaya fruit. Int. J. Food Microbiol. 2020, 326, 108649. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.M.; Quyen, N.T.T.; Thuy, D.T.K. Effect of edible coating and antifungal emulsion system on Colletotrichum acutatum and shelf life of strawberries. Vietnam. J. Chem. 2020, 58, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hortic. 2014, 34, 18–26. [Google Scholar] [CrossRef]
- Bosquez-Molina, E.; Ronquillo-de Jesús, E.; Bautista-Baños, S.; Verde-Calvo, J.R.; Morales-López, J. Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol. Technol. 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Oliveira, J.; Gloria, E.M.; Parisi, M.C.M.; Baggio, J.S.; Silva, P.P.M.; Ambrosio, C.M.S.; Spoto, M.H.F. Antifungal activity of essential oils associated with carboxymethylcellulose against Colletotrichum acutatum in strawberries. Sci. Hortic. 2019, 243, 261–267. [Google Scholar] [CrossRef]
- Ali, A.; Bordoh, P.K.; Singh, A.; Siddiqui, Y.; Droby, S. Post-harvest development of anthracnose in pepper (Capsicum spp): Etiology and management strategies. Crop Prot. 2016, 90, 132–141. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S.; Qazi, I.M.; Durrani, Y.; Sarkhosh, A.; Hussain, I.; Brecht, J.K. Pre-storage chitosan-thyme oil coating control anthracnose in mango fruit. Sci. Hortic. 2021, 284, 110139. [Google Scholar] [CrossRef]
- Boonruang, K.; Kerddonfag, N.; Chinsirikul, W.; Mitcham, E.J.; Chonhenchob, V. Antifungal effect of poly(lactic acid) films containing thymol and R-(−)-carvone against anthracnose pathogens isolated from avocado and citrus. Food Control 2017, 78, 85–93. [Google Scholar] [CrossRef]
- Grande Tovar, C.D.; Delgado-Ospina, J.; Navia Porras, D.P.; Peralta-Ruiz, Y.; Cordero, A.P.; Castro, J.I.; Chaves López, C. Colletotrichum gloesporioides inhibition in situ by chitosan-Ruta graveolens essential oil coatings: Effect on microbiological, physicochemical, and organoleptic properties of guava (Psidium guajava L.) during room temperature storage. Biomolecules 2019, 9, 399. [Google Scholar] [CrossRef] [Green Version]
- Hosseni, S.; Amini, J.; Rafei, J.N.; Khorshidi, J. Management of strawberry anthracnose using plant essential oils as bio-fungicides, and evaluation of their effects on quality of strawberry fruit. J. Oleo Sci. 2020, 69, 377–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkhosh, A.; Vargas, A.I.; Schaffer, B.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Postharvest management of anthracnose in avocado (Persea americana Mill.) fruit with plant-extracted oils. Food Packag. Shelf Life 2017, 12, 16–22. [Google Scholar] [CrossRef]
- Zillo, R.R.; da Silva, P.P.M.; de Oliveira, J.; da Gloria, E.M.; Spoto, M.H.F. Carboxymethylcellulose coating associated with essential oil can increase papaya shelf life. Sci. Hortic. 2018, 239, 70–77. [Google Scholar] [CrossRef]
- Madjouko, M.A.; Tchameni, S.N.; Tchinda, E.S.; Dongmo Jazet, P.M.; Kamsu, P.N.; Medzue Souop Kamga, V.A.; Sameza, M.L.; Tchoumbougnang, F.; Menut, C. Inhibitory effects of essential oils from Ocimum basilicum and Ocimum gratissimum on Colletotrichum musae: The causal agent of bananas anthracnose. J. Phytopathol. 2019, 167, 257–264. [Google Scholar] [CrossRef]
- Idris, F.M.; Ibrahim, A.M.; Forsido, S.F. Essential Oils to Control Colletotrichum musae in vitro and in vivo on Banana Fruits. Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 291–302. [Google Scholar]
- Duduk, N.; Markovic, T.; Vasic, M.; Duduk, B.; Vico, I.; Obradovic, A. Antifungal Activity of Three Essential Oils against Colletotrichum acutatum, the Causal Agent of Strawberry Anthracnose. J. Essent. Oil-Bear. Plants 2015, 18, 529–537. [Google Scholar] [CrossRef]
- Antonioli, G.; Fontanella, G.; Echeverrigaray, S.; Longaray Delamare, A.P.; Fernandes Pauletti, G.; Barcellos, T. Poly(lactic acid) nanocapsules containing lemongrass essential oil for postharvest decay control: In vitro and in vivo evaluation against phytopathogenic fungi. Food Chem. 2020, 326, 126997. [Google Scholar] [CrossRef]
- Khaliq, G.; Abbas, H.T.; Ali, I.; Waseem, M. Aloe vera gel enriched with garlic essential oil effectively controls anthracnose disease and maintains postharvest quality of banana fruit during storage. Hortic. Environ. Biotechnol. 2019, 60, 659–669. [Google Scholar] [CrossRef]
- Chavez-Magdaleno, M.E.; Gonzalez-Estrada, R.R.; Ramos-Guerrero, A.; PlascenciaJatomea, M.; Guti´errez-Martínez, P. Effect of pepper tree (Schinus molle) essential oil-loaded chitosan bio-nanocomposites on postharvest control of Colletotrichum gloeosporioides and quality evaluations in avocado (Persea americana) cv. Hass. Food Sci. Biotechnol. 2018, 27, 1871–1875. [Google Scholar] [CrossRef]
- Klangmuang, P.; Sothornvit, R. Active coating from hydroxypropyl methylcellulose-based nanocomposite incorporated with Thai essential oils on mango (cv. Namdokmai Sithong). Food Biosci. 2020, 23, 9–15. [Google Scholar] [CrossRef]
- Purkait, A.; Worede, R.E.; Baral, D.; Hazra, D.K.; Panja, B.N.; Biswas, P.K.; Kole, R.K. Development of nanoemulsion formulation of mustard oil, its chemical characterization and evaluation against post harvest anthracnose pathogens. Indian Phytopathol. 2020, 73, 449–460. [Google Scholar] [CrossRef]
- Feng, J.; Wang, R.; Chen, Z.; Zhang, S.; Yuan, S.; Cao, H.; Jafari, S.M.; Yang, W. Formulation optimization of D-limonene-loaded nanoemulsions as a natural and efficient biopesticide. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124746. [Google Scholar] [CrossRef]
- Felizardo, M.G.A.C.; Pereira, A.P.M.; de Lima, T.P.; de Sousa, T.L.; Guterres, C.V.F.; Oliveira, J.P.M.; Martins, T.G.T.; Filho, V.E.M.; Everton, G.O. Chemical profile, stability and fungicide activity of oil-in-water nanoemulsion (O/A) incorporated with Ba-har essential oil. Ciência Nat. 2021, 43, e57. [Google Scholar] [CrossRef]
- de Lima, T.P.; de Sousa, T.L.; Oliveira, J.P.M.; Felizardo, M.G.A.C.; Everton, G.O.; Mouchrek Filho, V.E. Chemical profile, thermodynamic stability and fungicidal activity of the nanoemulsion incorporated with essential oil and hydroalcoholic extract of Syzygium aromaticum (L.) Merr. & LM. Perry. Ciência Nat. 2021, 43, e77. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, T.S.; Costa, A.M.M.; Cabral, L.M.C.; Freitas-Silva, O.; Rosenthal, A.; Tonon, R.V. Anthracnose Controlled by Essential Oils: Are Nanoemulsion-Based Films and Coatings a Viable and Efficient Technology for Tropical Fruit Preservation? Foods 2023, 12, 279. https://doi.org/10.3390/foods12020279
de Oliveira TS, Costa AMM, Cabral LMC, Freitas-Silva O, Rosenthal A, Tonon RV. Anthracnose Controlled by Essential Oils: Are Nanoemulsion-Based Films and Coatings a Viable and Efficient Technology for Tropical Fruit Preservation? Foods. 2023; 12(2):279. https://doi.org/10.3390/foods12020279
Chicago/Turabian Stylede Oliveira, Tamires Sousa, André Mesquita Magalhães Costa, Lourdes Maria Corrêa Cabral, Otniel Freitas-Silva, Amauri Rosenthal, and Renata Valeriano Tonon. 2023. "Anthracnose Controlled by Essential Oils: Are Nanoemulsion-Based Films and Coatings a Viable and Efficient Technology for Tropical Fruit Preservation?" Foods 12, no. 2: 279. https://doi.org/10.3390/foods12020279