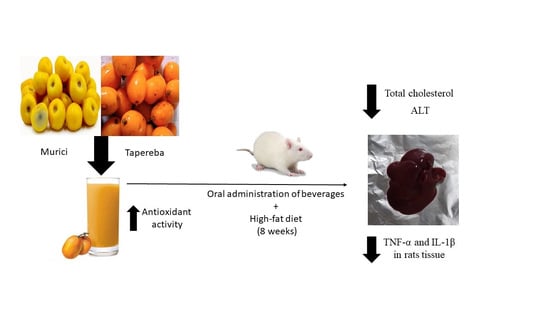
Murici (Byrsonima crassifolia (L.) Kunth and verbascifolia (L.)) and Tapereba (Spondias mombin) Improve Hepatic and Inflammatory Biomarkers in High-Fat-Diet Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Blood and Tissue Collection
2.3. Serum Analyses
2.4. Liver Cytokine Analyses
2.5. Antioxidant Activity in Plasma and Liver Tissue
2.5.1. DPPH Assay
2.5.2. ORAC Assay
2.6. Apoptosis Assay
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malta, L.G.; Ghiraldini, F.G.; Reis, R.; Oliveira, M.V.; Silva, L.B.; Pastore, G.M. In vivo analysis of antigenotoxic and antimutagenic properties of two Brazilian Cerrado fruits and the identification of phenolic phytochemicals. Food Res. Int. 2012, 49, 604–611. [Google Scholar] [CrossRef]
- Rufino, S.M.; Alves, R.E.; de Brito, E.S.; Pérez-jiménez, J.; Saura-calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Shanley, P.; Cymerys, M.; Serra, M.; Medina, G. Fruit Trees and Useful Plants in Amazonian Life, 2nd ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Fernandes, F.N.; Brito, E.S. Free radical scavenging behavior of ten exotic tropical fruits extracts. Food Res. Int. 2011, 44, 2072–2075. [Google Scholar] [CrossRef] [Green Version]
- Tiburski, J.H.; Rosenthal, A.; Deliza, R.; de Oliveira Godoy, R.L.; Pacheco, S. Nutritional properties of yellow mombin (Spondias mombin L.) pulp. Food Res. Int. 2011, 44, 2326–2331. [Google Scholar] [CrossRef] [Green Version]
- Souza, I.G.B.; Souza, V.A.B.; Lima, P.S.C. Molecular characterization of Platonia insignis Mart. (‘Bacurizeiro’) using inter simple sequence repeat (ISSR) markers. Mol. Biol. Rep. 2013, 40, 3835–3845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. World Health Organization: Taking Action on Childhood Obesity; World Health Organization: Geneva, Switzerland, 2017; pp. 1–8. [Google Scholar]
- Zhang, Q.; Zhou, P.H.; Zhou, X.L.; Zhang, D.L.; Gu, Q.; Zhang, S.J.; Zhang, J.; Zhang, J.S.; Qian, Z.Y. Effect of magnesium gluconate administration on lipid metabolism, antioxidative status, and related gene expression in rats fed a high-fat diet. Magnes. Res. 2018, 31, 117–130. [Google Scholar] [CrossRef]
- Wang, C.H.T. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Karppi, J.; Kurl, S.; Ronkainen, K.; Kauhanen, J.; Laukkanen, J.A. Serum Carotenoids Reduce Progression of Early Atherosclerosis in the Carotid Artery Wall among Eastern Finnish Men. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shiou, Y.L.; Huang, I.C.; Lin, H.T.; Lee, H.C. High fat diet aggravates atrial and ventricular remodeling of hypertensive heart disease in aging rats. J. Formos. Med. Assoc. 2018, 117, 621–631. [Google Scholar] [CrossRef]
- Tsai, H.E.; Liu, L.F.; Dusting, G.J.; Weng, W.T.; Chen, S.C.; Kung, M.L.; Tee, R.; Liu, G.S.; Tai, M.H. Pro-opiomelanocortin gene delivery suppresses the growth of established Lewis lung carcinoma through a melanocortin-1 receptor-independent pathway. J. Gene Med. 2012, 14, 44–53. [Google Scholar] [CrossRef]
- De Souza, V.R.; Aniceto, A.; Abreu, J.P.; Montenegro, J.; Bonquipani, B.; de Jesuz, V.A.; De Barros Elias Campos, M.; Marcellini, P.S.; Freitas-Silva, Q.; Silva Cadena, R.; et al. Fruit-based drink sensory, physicochemical and antioxidant properties in the Amazon region: Murici (Byrsonima crassifolia (L.) Kunth and verbascifolia (L.) DC) and tapereba (Spondia mombin). Food Sci. Nutr. 2020, 8, 2341–2347. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, M.E.L.; Xavier, A.R.; Azeredo, V.B. Diet and liver apoptosis in rats: A particular metabolic pathway. Nutr. Hosp. 2017, 34, 463. [Google Scholar] [CrossRef]
- Brand-Wiliams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Deji, N.; Kume, S.; Araki, S.; Soumura, M.; Sugimoto, T.; Isshisaki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Koya, D.; Haneda, M.; et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am. J. Physiol. Ren. Physiol. 2009, 296, 118–126. [Google Scholar] [CrossRef] [Green Version]
- El-Sayyad, H.I.H.; El-Shershaby, E.M.F.; El-Mansi, A.A.; El-Ashry, N.E. Anti-hypercholesterolemic impacts of barley and date palm fruits on the ovary of Wistar albino rats and their offspring. Reprod. Biol. 2018, 18, 236–251. [Google Scholar] [CrossRef]
- Baptista Alves Faria, S.; Rosse de Souza, V.; Furtado Dias, J. Effect of grape juice consumption on antioxidant activity and interleukin-6 concentration in lactating rats. Nutr. Hosp. 2016, 33, 1418–1423. [Google Scholar]
- Costa Júnior, J.; Ferraz, A.B.F.; Sousa, T.O.; Silva, R.A.C.; De Lima, S.; Feitosa, C.M.; Citó, A.; Melo Cavalcante, A.; Freitas, R.M.; Moura Sperotto, A.R.; et al. Investigation of Biological Activities of Dichloromethane and Ethyl Acetate Fractions of Platonia insignis Mart. Seed. Basic Clin. Pharmacol. Toxicol. 2013, 112, 34–41. [Google Scholar] [CrossRef]
- Aniceto, A.; Porte, A.; Montenegro, J.; Cadena, R.S.; Teodoro, A.J. A review of the fruit nutritional and biological activities of three Amazonian species: Bacuri (Platonia insignis), murici (Byrsonima spp.), and taperebá (Spondias mombin). Fruits 2017, 72, 317–326. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Sacanella, I.; Mitjavila, M.T.; Lamuela-Raventos, R.M.; Moreno, J.J. Bioactive Compounds of Cooked Tomato Sauce Modulate Oxidative Stress and Arachidonic Acid Cascade Induced by Oxidized LDL in Macrophage Cultures. Nutrients 2019, 11, 1880. [Google Scholar] [CrossRef] [Green Version]
- Mian, A.N.; Schwartz, G.J. Measurement and Estimation of Glomerular Filtration Rate in Children. Adv. Chronic Kidney Dis. 2017, 24, 348–356. [Google Scholar] [CrossRef]
- Madkour, F.F.; Abdel-Daim, M.M. Hepatoprotective and antioxidant activity of Dunaliella salina in paracetamol-induced acute toxicity in rats. Indian J. Pharm. Sci. 2013, 75, 642–648. [Google Scholar] [PubMed]
- Toledo, R.C.L.; Brito, L.F.; Ribeiro, S.M.R.; Peluzio, M.C.G.; de Siqueira, C.L.M.; de Queiroz, J.H. Efeito da ingestão da polpa de manga (Mangifera indica L.) sobre os parâmetros bioquímicos séricos e integridade hepática em ratos. Biosci. J. 2013, 29, 516–525. [Google Scholar]
- Jimenez-Escobar, M.; Pascual-Mathey, L.; Beristain, C.; Flores-Andrade, E.; Jiménez, M. In vitro and In vivo antioxidant properties of paprika carotenoids nanoemulsions. LWT 2019, 118, 108694. [Google Scholar] [CrossRef]
- De Jesuz, V.A.; de Barros Elias Campos, M.; Rosse de Souza, V.; Palmiciano Bede, T.; Portugal Tavares de Moraes, B.; Silva, R.A.; Gonçalves de Albuquerque, C.F.; Blondet de Azeredo, V.; Teodoro, A.J. Lycopene and Tomato Sauce Improve Hepatic and Cardiac Cell Biomarkers in Rats. J. Med. Food 2019, 22, 1175–1182. [Google Scholar] [CrossRef]
- Fialho, E.; Kremer Faller, A.L. Polyphenol availability in fruits. Rev. Saúde Pública 2009, 43, 211–218. [Google Scholar]
- Storniolo, C.E.; Sacanella, I.; Lamuela-Raventos, R.M.; Moreno, J.J. Bioactive Compounds of Mediterranean Cooked Tomato Sauce (Sofrito) Modulate Intestinal Epithelial Cancer Cell Growth Through Oxidative Stress/Arachidonic Acid Cascade Regulation. Omega 2020, 5, 17071–17077. [Google Scholar] [CrossRef]
- De Souza, V.R.; Concentino Menezes Brum, M.; dos Santos Guimarãeset, I.; dos Santos Guimarães, P.; do Amaral, T.O.; Pimentel Abreual, J.; Passos, T.; Freitas-Silva, O.; Pereira Gimba, E.R.; Junger Teodoro, A.; et al. Amazon fruits inhibit growth and promote pro-apoptotic effects on human ovarian carcinoma cell lines. Biomolecules 2019, 9, 707. [Google Scholar] [CrossRef] [Green Version]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Yuzefovych, L.V.; Musiyenko, S.I.; Wilson, G.L.; Rachek, L.I. Mitochondrial DNA Damage and Dysfunction, and Oxidative Stress Are Associated with Endoplasmaic Reticulum Stress, Protein Degradation and Apoptosis in High Fat Diet-Induced Insulin Resistance Mice. PLoS ONE 2013, 8, e54059. [Google Scholar] [CrossRef]





| Components (g/Kg) | AIN-93 G | High-Fat Diet |
|---|---|---|
| Casein | 200.00 | 200.00 |
| Starch | 397.49 | 397.49 |
| Dextrin | 132.00 | 132.00 |
| Sucrose | 100.00 | 100.00 |
| L-cystine | 3.00 | 3.00 |
| Soy oil | 70.00 | 70.00 |
| Hydrogenated fat | 0 | 200.00 |
| Mixture of salts (AIN-93G) | 35.00 | 35.00 |
| Vitamin Blend (AIN-93G) | 10.00 | 10.00 |
| Microcellulose | 50.00 | 50.00 |
| Choline | 2.500 | 2.500 |
| Parameters | CON | HF | Mu-HF | Tap-HF | MT-HF |
|---|---|---|---|---|---|
| Initial body weight (g) | 181.20 ± 9.63 | 180.30 ± 10.05 | 182.30 ± 9.42 | 183.00 ± 6.45 | 180.80 ± 10.06 |
| Weight gain (g) | 76.20 ± 6.30 | 88.50 ± 8.42 | 87.50 ± 15.28 | 80.80 ± 15.79 | 79.75 ± 12.69 |
| Feed consumption/group(g) | 1040.54 ± 90.59 | 978.00 ± 49.72 | 851.87 ± 46.32 * | 868.50 ± 123.82 * | 811.75 ± 87.63 * |
| Dietary energy intake (Kcal/Week) | 4105.71 ± 356.89 | 5621.54 ± 286.40 | 4896.58 ± 266.28 | 4992.14 ± 711.77 | 4665.94 ± 503.75 |
| Water consumption/group (mL) | 2240.00 ± 330.50 | 2448.60 ± 423.66 | 1705.00 ± 525.22 * | 1784.00 ± 139.92 * | 1788.25 ± 302.81 * |
| Beverage consumption/group (mL) | − | − | 761.08 ± 48.13 * | 893.34 ± 41.58 | 997.27 ± 43.95 |
| Liver weight (g) | 7.54 ± 1.00 | 7.70 ± 1.08 | 7.64 ± 1.18 | 8.16 ± 1.02 | 6.87 ± 0.80 |
| Hepatosomatic index | 2.93 ± 0.95 | 2.87 ± 0.42 | 2.83 ± 0.39 | 3.10 ± 0.45 | 2.63 ± 0.18 |
| Parameters | CON | HF | Mu-HF | Tap-HF | MT-HF |
|---|---|---|---|---|---|
| Glycemia (mg/dL) | 111.4 ± 4.04 | 109.20 ± 15.09 | 109.50 ± 23.44 | 114.55 ± 5.50 | 97.00 ± 7.87 * |
| Progesterone (ng/mL) | 2.58 ± 0.34 | 2.51 ± 0.35 | 2.26 ± 0.75 | 2.49 ± 0.37 | 2.34 ± 0.28 |
| Total cholesterol (mg/dL) | 46.67 ± 5.69 | 66.00 ± 6.68 * | 50.75 ± 9.84 | 58.00 ± 5.24 * | 57.00 ± 3.80 * |
| Triglycerides (mg/dL) | 59.40 ± 15.34 | 36.0 ± 10.98 | 54.80 ± 11.14 | 48.60 ± 10.31 | 55.60 ± 16.30 |
| HDL (mg/dL) | 23.40 ± 3.58 | 25.60 ± 3.50 | 23.60 ± 5.03 | 26.80 ± 3.11 | 25.20 ± 1.48 |
| AST (UK) | 177.50 ± 30.84 | 163.00 ± 3.0 | 174.60 ± 39.64 | 182.00 ± 35.74 | 168.00 ± 29.0 |
| ALT (U/mL) | 37.60 ± 4.22 | 42.00 ± 7.34 * | 30.75 ± 3.30 | 39.50 ± 2.64 ** | 34.75 ± 3.20 ** |
| Urea (mg/dL) | 36.50 ± 5.26 | 31.80 ± 4.86 | 29.20 ± 2.58 | 31.60 ± 3.28 | 34.00 ± 3.16 |
| Creatinine (mg/dL) | 0.40 ± 0.08 | 0.52 ± 0.04 | 0.46 ± 0.05 | 0.48 ± 0.08 | 0.54 ± 0.05 |
| Calcium (mg/dl) | 7.64 ± 0.45 | 7.77 ± 0.18 | 7.70 ± 0.47 | 8.90 ± 0.90 * | 7.60 ± 0.55 |
| Magnesium | 1.12 ± 0.18 | 0.94 ± 0.19 | 0.94 ± 0.18 | 0.84 ± 0.11 | 0.68 ± 0.13 |
| Phosphorus (mg/dL) | 4.26 ± 0.62 | 4.02 ± 0.65 | 3.60 ± 0.31 * | 4.44 ± 0.71 ** | 4.74 ± 0.35 ** |
| Albumin (g/dL) | 2.42 ± 0.16 | 2.64 ± 0.19 | 2.78 ± 0.11 | 2.85 ± 0.35 | 2.42 ± 0.13 |
| Protein (µg/L) | 5.10 ± 0.36 | 5.08 ± 0.24 | 5.42 ± 0.24 | 5.44 ± 0.57 | 5.02 ± 0.22 |
| Parameters | CON | HF | Mu-HF | Tap-HF | MT-HF |
|---|---|---|---|---|---|
| DPPH assay (% reduction) | 34.76 ± 5.00 | 33.52 ± 8.92 | 51.38 ± 7.94 * | 51.85 ± 12.81 * | 50.92 ± 2.39 * |
| ORAC assay (μM TE/g) | 196.64 ± 95.91 | 151.23 ± 37.35 | 166.15 ± 13.08 | 461.14 ± 96.07 * | 815.34 ± 125.09 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, V.R.; Lima, T.P.B.; Bedê, T.P.; Faria, S.B.A.; Alves, R.; Louzada, A.; de Moraes, B.P.T.; Silva, A.R.; Gonçalves de Albuquerque, C.F.; de Azeredo, V.B.; et al. Murici (Byrsonima crassifolia (L.) Kunth and verbascifolia (L.)) and Tapereba (Spondias mombin) Improve Hepatic and Inflammatory Biomarkers in High-Fat-Diet Rats. Foods 2023, 12, 255. https://doi.org/10.3390/foods12020255
de Souza VR, Lima TPB, Bedê TP, Faria SBA, Alves R, Louzada A, de Moraes BPT, Silva AR, Gonçalves de Albuquerque CF, de Azeredo VB, et al. Murici (Byrsonima crassifolia (L.) Kunth and verbascifolia (L.)) and Tapereba (Spondias mombin) Improve Hepatic and Inflammatory Biomarkers in High-Fat-Diet Rats. Foods. 2023; 12(2):255. https://doi.org/10.3390/foods12020255
Chicago/Turabian Stylede Souza, Vanessa Rosse, Thuane Passos Barbosa Lima, Teresa Palmiciano Bedê, Sabrina Baptista Alves Faria, Renata Alves, Alana Louzada, Bianca Portugal Tavares de Moraes, Adriana Ribeiro Silva, Cassiano Felippe Gonçalves de Albuquerque, Vilma Blondet de Azeredo, and et al. 2023. "Murici (Byrsonima crassifolia (L.) Kunth and verbascifolia (L.)) and Tapereba (Spondias mombin) Improve Hepatic and Inflammatory Biomarkers in High-Fat-Diet Rats" Foods 12, no. 2: 255. https://doi.org/10.3390/foods12020255