Surface Modification via Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD–ACP): Improved Functional Properties of Soy Protein Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
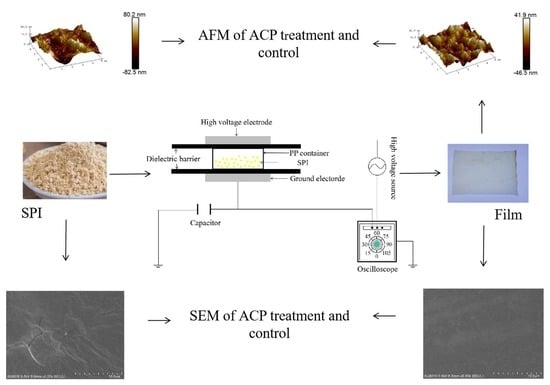
2.2. ACP Treatment of Soy Protein Solution
2.3. Preparation of Soy Protein Film
2.4. Mechanical Properties of Soy Protein Film
2.5. Water Vapor Permeability (WVP)
2.6. Moisture Content (MC)
2.7. Solubility (So)
2.8. Swelling Index (Si)
2.9. Differential Scanning Calorimetry (DSC)
2.10. Scanning Electron Microscopy (SEM)
2.11. Fourier Transform Infrared Spectroscopy (FTIR)
2.12. Atomic Force Microscopy (AFM)
2.13. Water Contact Angle (WCA)
2.14. Statistical Analysis
3. Results
3.1. Factors Affecting Elongation at Break and Water Vapor Permeability of the Soy Protein Film
3.1.1. Effect of Soybean Protein Concentration
3.1.2. Effect of Glycerol Concentration
3.2. Effects of Different ACP Treatment Voltages on Soy Protein Film
3.3. Effects of Different ACP Treatment Times on Soy Protein Film
3.4. Solubility, Swelling Index, and Moisture Content of Soy Protein Film
3.5. Thermal Properties of Soy Protein Film
3.6. Microstructure of Soy Protein Film
3.7. Fourier Transform Infrared Spectroscopy Analysis of Soy Protein Film
3.8. Surface Roughness of Soy Protein Film
3.9. Surface Hydrophilicity of Soy Protein Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DBD–ACP | Dielectric barrier discharge Atmospheric cold plasma |
| DBD | Dielectric barrier discharge |
| ACP | Atmospheric cold plasma |
| CP | Cold plasma |
| WVP | Water vapor permeability |
| MC | Moisture content |
| So | Solubility |
| Si | Swelling index |
| DSC | Differential scanning calorimetry |
| Td | Denaturation temperature |
| Tg | Glass transition temperature |
| SEM | Scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| Rmax | Maximum roughness |
| Rms | Root mean square roughness |
References
- Kontominas, M.G. Use of alginates as food packaging materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, T.; Cao, W.; Wu, Y.; Chi, Y.; Zhang, H.; Liu, Y. Properties of soy protein isolate antimicrobial films and its application in preservation of meat. Emir. J. Food Agric. 2017, 29, 589–600. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, H.; Lee, J.Y.; Ramachandraiah, K.; Hong, G.P. β-Cyclodextrin-mediated beany flavor masking and textural modification of an isolated soy protein-based yuba film. Foods 2020, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.Y.; Song, F.; Zhang, M.; Wang, X.L.; Wang, Y.Z. Soy protein isolate films with improved property via a facile surface coating. Ind. Crops Prod. 2014, 54, 102–108. [Google Scholar] [CrossRef]
- Kumar, R.; Zhang, L. Soy protein films with the hydrophobic surface created through non-covalent interactions. Ind. Crops Prod. 2009, 29, 485–494. [Google Scholar] [CrossRef]
- Cho, S.Y.; Rhee, C. Sorption characteristics of soy protein films and their relation to mechanical properties. LWT-Food Sci. Technol. 2002, 35, 151–157. [Google Scholar] [CrossRef]
- Rani, S.; Singh, A.K.; Paswan, R.R.; Kumar, K.D.; Kumar, R. Preparation, characterization and antibacterial evaluation of soy protein isolate biopolymeric films loaded with nalidixic acid. J. Polym. Environ. 2020, 28, 1841–1850. [Google Scholar] [CrossRef]
- Kumar, R.; Rani, P.; Dinesh Kumar, K. Soy protein isolate film by incorporating mandelic acid as Well as through fermentation mediated by Bacillus Subtilis. J. Renew. Mater. 2019, 7, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Bosquez-Molina, E.; Tomás, S.A.; Rodríguez-Huezo, M.E. Influence of CaCl2 on the water vapor permeability and the surface morphology of mesquite gum based edible films. LWT-Food Sci. Technol. 2010, 43, 1419–1425. [Google Scholar] [CrossRef]
- Kumar, R.; Praveen, R.; Rani, S.; Sharma, K.; Tiwary, P.K.; Dinesh Kumar, K. ZnSe nanoparticles reinforced biopolymeric soy protein isolate film. J. Renew. Mater. 2019, 7, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Anjum, K.N.; Rani, S.; Sharma, K.; Tiwary, K.P.; Kumar, K.D. Material properties of ZnS nanoparticles incorporated soy protein isolate biopolymeric film. Plast. Rubber Compos. 2019, 48, 448–456. [Google Scholar] [CrossRef]
- Vieira, T.M.; Moldão-Martins, M.; Alves, V.D. Design of chitosan and alginate emulsion-based formulations for the production of monolayer crosslinked edible films and coatings. Foods 2021, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Barać, M.; Stanojević, S.; Jovanović, S.; Pešić, M. Soy protein modification: A review. Acta Period. Technol. 2004, 35, 3–16. [Google Scholar] [CrossRef]
- Wang, J.M.; Yang, X.Q.; Yin, S.W.; Zhang, Y.; Tang, C.H.; Li, B.S.; Yuan, D.B.; Guo, J. Structural rearrangementof ethanol-denatured soy proteins by high hydrostatic pressure treatment. J. Agric. Food Chem. 2011, 59, 7324–7332. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Van Cleynenbreugel, R.; Boehm, D.; Bourke, P. Assessing the biological safety of atmospheric cold plasma treated wheat using cell and insect models. Foods 2020, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. 2015, 29, 247–269. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Muhammad, A.I.; Suo, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Application of a dielectric barrier discharge atmospheric cold plasma (Dbd-Acp) for eshcerichia coli inactivation in apple juice. J. Food Sci. 2018, 83, 401–408. [Google Scholar] [CrossRef]
- Trevisani, M.; Berardinelli, A.; Cevoli, C.; Cecchini, M.; Ragni, L.; Pasquali, F. Effects of sanitizing treatments with atmospheric cold plasma, SDS and lactic acid on verotoxin-producing Escherichia coli and Listeria monocytogenes in red chicory (radicchio). Food Control 2017, 78, 138–143. [Google Scholar] [CrossRef]
- Timmons, C.; Pai, K.; Jacob, J.; Zhang, G.; Ma, L.M. Inactivation of Salmonella enterica, Shiga toxin-producing Escherichia coli, and Listeria monocytogenes by a novel surface discharge cold plasma design. Food Control 2018, 84, 455–462. [Google Scholar] [CrossRef]
- Chingsungnoen, A.; Maneerat, S.; Chunpeng, P.; Poolcharuansin, P.; Nam-Matra, R. Antimicrobial treatment of Escherichia coli and Staphylococcus aureus in herbal tea using low-temperature plasma. J. Food Protect. 2018, 81, 1503–1507. [Google Scholar] [CrossRef]
- Patange, A.; Boehm, D.; Bueno-Ferrer, C.; Cullen, P.J.; Bourke, P. Controlling Brochothrix thermosphacta as a spoilage risk using in-package atmospheric cold plasma. Food Microbiol. 2017, 66, 48–74. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Andrés, J.M.; Álvarez, C.; Cullen, P.J.; Tiwari, B.K. Effect of cold plasma on the techno-functional properties of animal protein food ingredients. Innov. Food Sci. Emerg. 2019, 58, 102205. [Google Scholar] [CrossRef]
- Bußler, S.; Steins, V.; Ehlbeck, J.; Schlüter, O. Impact of thermal treatment versus cold atmospheric plasma processing on the techno-functional protein properties from Pisum sativum ‘Salamanca’. J. Food Eng. 2015, 167, 166–174. [Google Scholar] [CrossRef]
- Chaple, S.; Sarangapani, C.; Jones, J.; Carey, E.; Causeret, L.; Genson, A.; Duffy, B.; Bourke, P. Effect of atmospheric cold plasma on the functional properties of whole wheat (Triticum aestivum L.) grain and wheat flour. Innov. Food Sci. Emerg. 2020, 66, 102529. [Google Scholar] [CrossRef]
- Ji, H.; Han, F.; Peng, S.; Yu, J.; Li, L.; Liu, Y.; Chen, Y.; Li, S.; Chen, Y. Behavioral solubilization of peanut protein isolate by atmospheric pressure cold plasma (ACP) treatment. Food Bioprocess Technol. 2019, 12, 2018–2027. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Jiménez, A.; Bourke, P.; Cullen, P.J. Characterization of polylactic acid films for food packaging as affected by dielectric barrier discharge atmospheric plasma. Innov. Food Sci. Emerg. 2014, 21, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Gao, A.; Zhao, Y.; Li, Y.T.; Chen, Y. Characterization of physicochemical and structural properties of atmospheric cold plasma (ACP) modified zein. Food Bioprod Process. 2017, 106, 65–74. [Google Scholar] [CrossRef]
- Oh, Y.A.; Roh, S.H.; Min, S.C. Cold plasma treatments for improvement of the applicability of defatted soybean meal-based edible film in food packaging. Food Hydrocoll. 2016, 58, 150–159. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.G.; Sun, D.W.; Cheng, J.H. A review on recent advances in cold plasma technology for the food industry: Current applications and future trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.Z.; Chen, J.Y.; Chen, D.Z.; Deng, S.G.; Xu, B. Effect of cold plasma on maintaining the quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. J. Sci. Food Agric. 2019, 99, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Wiles, J.L.; Vergano, P.J.; Barron, F.H.; Bunn, J.M.; Testin, R.F. Water vapor transmission rates and sorption behavior of chitosan films. J. Food Sci. 2000, 65, 1175–1179. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Li, H.; Chi, Y.; Zhang, H.; Xia, N.; Ma, Y.; Jiang, L.; Zhang, X. Changes in properties of soy protein isolate edible films stored at different temperatures: Studies on water and glycerol migration. Foods 2021, 10, 1797. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Paglione, I.; Galindo, M.V.; de Souza, K.C.; Yamashita, F.; Grosso, C.R.F.; Sakanaka, L.S.; Shirai, M.A. Optimization of the conditions for producing soy protein isolate films. Emir. J. Food Agric. 2019, 31, 297–303. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Jahromi, M.; Niakousari, M.; Golmakani, M.T.; Ajalloueian, F.; Khalesi, M. Effect of dielectric barrier discharge atmospheric cold plasma treatment on structural, thermal and techno-functional characteristics of sodium caseinate. Innov. Food Sci. Emerg. 2020, 66, 102542. [Google Scholar] [CrossRef]
- Chen, G.; Dong, S.; Zhao, S.; Li, S.; Chen, Y. Improving functional properties of zein film via compositing with chitosan and cold plasma treatment. Ind. Crops Prod. 2019, 129, 318–326. [Google Scholar] [CrossRef]
- Dong, S.; Guo, P.; Chen, Y.; Chen, G.Y.; Ji, H.; Ran, Y.; Li, S.H.; Chen, Y. Surface modification via atmospheric cold plasma (ACP): Improved functional properties and characterization of zein film. Ind. Crops Prod. 2018, 115, 124–133. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.k. Cold plasma treatment of dairy proteins in relation to functionality enhancement. Trends Food Sci. Technol. 2020, 102, 30–36. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Y.; Jin, N.; Li, J.; Dong, S.; Li, S.; Zhang, Z.; Chen, Y. Zein films with porous polylactic acid coatings via cold plasma pre-treatment. Ind. Crops Prod. 2020, 150, 112382. [Google Scholar] [CrossRef]
- Dong, S.; Wang, J.M.; Cheng, L.M.; Lu, Y.L.; Li, S.H.; Chen, Y. Behavior of zein in aqueous ethanol under atmospheric pressure cold plasma treatment. J. Agric. Food Chem. 2017, 65, 7352–7360. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Bourke, P.; Cullen, P.J. Effects of cold plasma on surface, thermal and antimicrobial release properties of chitosan film. J. Renew. Mater. 2017, 5, 14–20. [Google Scholar] [CrossRef]
- Karam, L.; Casetta, M.; Chihib, N.E.; Bentiss, F.; Maschke, U.; Jama, C. Optimization of cold nitrogen plasma surface modification process for setting up antimicrobial low density polyethylene films. J. Taiwan Inst. Chem. E 2016, 64, 299–305. [Google Scholar] [CrossRef]










| Stage | So (%) | Si (%) | MC (%) |
|---|---|---|---|
| Control | 34.15 ± 0.07 | 134.89 ± 0.41 | 25.13 ± 0.16 |
| ACP treatment | 37.35 ± 0.15 | 153.52 ± 1.32 | 31.44 ± 0.21 |
| Thermodynamic Parameters | Tg (°C) | Td (°C) | Peak Area (μVs/mg) | Peak (μV/mg) | Enthalpy (J/g) |
|---|---|---|---|---|---|
| Control | 98.2 | 113.4 | 512.6 | 3.7829 | 10.338 |
| ACP treatment | 100.3 | 123.8 | 674.9 | 4.9177 | 14.025 |
| Roughness Index | Rmax (nm) | Ra (nm) | Rms (nm) |
|---|---|---|---|
| Control | 162.7 ± 2.14 | 17.1 ± 0.45 | 22.0 ± 0.54 |
| ACP treatment | 88.4 ± 1.33 | 10.9 ± 27 | 13.4 ± 0.19 |
| Water Contact Angle | Right | Left |
|---|---|---|
| Control | 86.9° ± 0.53 | 87.9° ± 0.47 |
| ACP treatment | 78.7° ± 0.32 | 77.2° ± 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Deng, S.; Chen, J. Surface Modification via Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD–ACP): Improved Functional Properties of Soy Protein Film. Foods 2022, 11, 1196. https://doi.org/10.3390/foods11091196
Li Z, Deng S, Chen J. Surface Modification via Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD–ACP): Improved Functional Properties of Soy Protein Film. Foods. 2022; 11(9):1196. https://doi.org/10.3390/foods11091196
Chicago/Turabian StyleLi, Zhibing, Shanggui Deng, and Jing Chen. 2022. "Surface Modification via Dielectric Barrier Discharge Atmospheric Cold Plasma (DBD–ACP): Improved Functional Properties of Soy Protein Film" Foods 11, no. 9: 1196. https://doi.org/10.3390/foods11091196