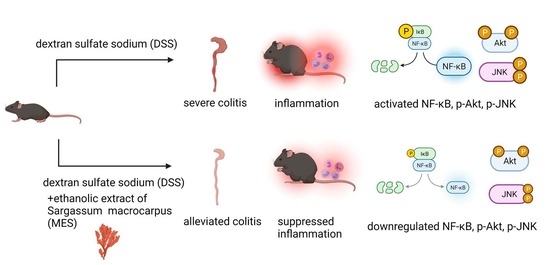
Meroterpenoid-Rich Ethanoic Extract of Sargassum macrocarpum Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of MES and Analysis of Chemical Components
2.3. Animals and Experimental Design
2.4. Dosage Information
2.5. Disease Activity Index (DAI) Score
2.6. Histopathological Analysis
2.7. Immunohistochemical Staining
2.8. Myeloperoxidase (MPO) Activity Assay
2.9. Measurement of Cytokines and Chemokines in Serum
2.10. Western Blot
2.11. Statistical Analysis
3. Results
3.1. MES Ameliorated DSS-Induced Colitis
3.2. MES Ameliorated Histological Damage in Colon Tissue and Inhibited Infiltration of Inflammatory Cells
3.3. MES Suppressed the Release of Inflammatory Molecules in Colon Tissue and Serum
3.4. MES Suppressed Signaling Proteins in Colon Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Cipriani, S.; Francisci, D.; Santucci, L.; Baldelli, F.; Distrutti, E.; Fiorucci, S. Highly specific blockade of CCR5 inhibits leukocyte trafficking and reduces mucosal inflammation in murine colitis. Sci. Rep. 2016, 6, 30802. [Google Scholar] [CrossRef] [Green Version]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42, quiz e30. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.S.; Cha, J.M.; Lee, H.H.; Choi, Y.S.; Seo, S.I.; Ko, K.J.; Park, D.I.; Kim, S.H.; Kim, T.J. Emerging trends of inflammatory bowel disease in South Korea: A nationwide population-based study. J. Gastroenterol Hepatol 2019, 34, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Nanau, R.M. Inflammatory bowel disease: Role of diet, microbiota, life style. Transl. Res. J. Lab. Clin. Med. 2012, 160, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Curtis, V.F.; Lanis, J.M.; Glover, L.E. Metabolic regulation of intestinal epithelial barrier during inflammation. Tissue Barriers 2015, 3, e970936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Meier, J.; Sturm, A. Current treatment of ulcerative colitis. World J. Gastroenterol. 2011, 17, 3204–3212. [Google Scholar] [CrossRef]
- Ibrahim, A.; Aziz, M.; Hassan, A.; Mbodji, K.; Collasse, E.; Coeffier, M.; Bounoure, F.; Savoye, G.; Dechelotte, P.; Marion-Letellier, R. Dietary alpha-linolenic acid-rich formula reduces adhesion molecules in rats with experimental colitis. Nutrition 2012, 28, 799–802. [Google Scholar] [CrossRef]
- Ghosh, S.; Panaccione, R. Anti-adhesion molecule therapy for inflammatory bowel disease. Ther. Adv. Gastroenterol 2010, 3, 239–258. [Google Scholar] [CrossRef] [Green Version]
- Naito, Y.; Yoshikawa, T. Role of matrix metalloproteinases in inflammatory bowel disease. Mol. Asp. Med. 2005, 26, 379–390. [Google Scholar] [CrossRef] [PubMed]
- White, W.L.; Wilson, P. World seaweed utilization. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 7–25. [Google Scholar]
- Rajapakse, N.; Kim, S.-K. Nutritional and digestive health benefits of seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar] [PubMed]
- Brownlee, I.; Fairclough, A.; Hall, A.; Paxman, J. The potential health benefits of seaweed and seaweed extract. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses. Marine Biology: Earth Sciences in the 21st Century; Nova Science Publishers: New York, NY, USA, 2012; pp. 119–136. [Google Scholar]
- Ko, S.J.; Bu, Y.; Bae, J.; Bang, Y.M.; Kim, J.; Lee, H.; Beom-Joon, L.; Hyun, Y.H.; Park, J.W. Protective effect of Laminaria japonica with probiotics on murine colitis. Mediat. Inflamm. 2014, 2014, 417814. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Amu, S.; Saunders, S.P.; Fallon, P.G. A mineral extract from red algae ameliorates chronic spontaneous colitis in IL-10 deficient mice in a mouse strain dependent manner. Phytother. Res. PTR 2014, 28, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Choi, A.-H.; Kwon, M.; Joung, E.-J.; Shin, T.; Lee, S.-G.; Kim, N.-G.; Kim, H.-R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef]
- Joung, E.J.; Cao, L.; Lee, B.; Gwon, W.G.; Park, S.H.; Kim, H.R. Sargahydroquinoic Acid, a Cyclooxygenase-2 Inhibitor, Attenuates Inflammatory Responses by Regulating NF-kappaB Inactivation and Nrf2 Activation in Lipopolysaccharide-Stimulated Cells. Inflammation 2021, 44, 2120–2131. [Google Scholar] [CrossRef]
- Joung, E.J.; Lee, B.; Gwon, W.G.; Shin, T.; Jung, B.M.; Yoon, N.Y.; Choi, J.S.; Oh, C.W.; Kim, H.R. Sargaquinoic acid attenuates inflammatory responses by regulating NF-kappaB and Nrf2 pathways in lipopolysaccharide-stimulated RAW 264.7 cells. Int. Immunopharmacol. 2015, 29, 693–700. [Google Scholar] [CrossRef]
- Gwon, W.G.; Joung, E.J.; Kwon, M.S.; Lim, S.J.; Utsuki, T.; Kim, H.R. Sargachromenol protects against vascular inflammation by preventing TNF-alpha-induced monocyte adhesion to primary endothelial cells via inhibition of NF-kappaB activation. Int. Immunopharmacol. 2017, 42, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gwon, W.-G.; Lee, B.; Joung, E.-J.; Choi, M.-W.; Yoon, N.; Shin, T.; Oh, C.-W.; Kim, H.-R. Sargaquinoic Acid Inhibits TNF-alpha-Induced NF-kappaB Signaling, Thereby Contributing to Decreased Monocyte Adhesion to Human Umbilical Vein Endothelial Cells (HUVECs). J. Agric. Food Chem. 2015, 63, 9053–9061. [Google Scholar] [CrossRef]
- Joung, E.J.; Kwon, M.; Gwon, W.G.; Cao, L.; Lee, S.G.; Utsuki, T.; Wakamatsu, N.; Kim, J.; Kim, H.-R. Meroterpenoid-Rich Fraction of the Ethanol Extract of Sargassum serratifolium Suppresses Collagen-Induced Rheumatoid Arthritis in DBA/1J Mice Via Inhibition of Nuclear Factor kappaB Activation. Mol. Nutr. Food Res. 2020, 64, e1900373. [Google Scholar] [CrossRef] [PubMed]
- Gwon, W.-G.; Joung, E.-J.; Shin, T.; Utsuki, T.; Wakamatsu, N.; Kim, H.-R. Meroterpinoid-rich fraction of the ethanol extract from Sargassum serratifolium suppresses TNF-α-induced monocytes adhesion to vascular endothelium and vascular inflammation in high cholesterol-fed C57BL/6J mice. J. Funct. Foods 2018, 46, 384–393. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Rashid, F.; Bragg, J.; Ibdah, J.A. Role of the JNK signal transduction pathway in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lim, S.-J.; Joung, E.-J.; Lee, B.; Oh, C.-W.; Kim, H.-R. Meroterpenoid-rich fraction of an ethanolic extract from Sargassum serratifolium alleviates obesity and non-alcoholic fatty liver disease in high fat-fed C57BL/6J mice. J. Funct. Foods 2018, 47, 288–298. [Google Scholar] [CrossRef]
- El-Medany, A.; Mahgoub, A.; Mustafa, A.; Arafa, M.; Morsi, M. The effects of selective cyclooxygenase-2 inhibitors, celecoxib and rofecoxib, on experimental colitis induced by acetic acid in rats. Eur. J. Pharmacol 2005, 507, 291–299. [Google Scholar] [CrossRef]
- Sheta, N.M.; Boshra, S.A. Fabrication and Evaluation of Celecoxib Oral Oleogel to Reduce the Inflammation of Ulcerative Colitis. AAPS PharmSciTech 2021, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, W.; Hong, S.; Park, H.; Yum, S.; Yoon, J.H.; Jung, Y. Colon-targeted celecoxib ameliorates TNBS-induced rat colitis: A potential pharmacologic mechanism and therapeutic advantages. Eur. J. Pharmacol 2014, 726, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Cuzzocrea, S.; Mazzon, E.; Serraino, I.; Dugo, L.; Centorrino, T.; Ciccolo, A.; Sautebin, L.; Caputi, A.P. Celecoxib, a selective cyclo-oxygenase-2 inhibitor reduces the severity of experimental colitis induced by dinitrobenzene sulfonic acid in rats. Eur. J. Pharmacol. 2001, 431, 91–102. [Google Scholar] [CrossRef]
- Joung, E.-J.; Gwon, W.-G.; Shin, T.; Jung, B.-M.; Choi, J.; Kim, H.-R. Anti-inflammatory action of the ethanolic extract from Sargassum serratifolium on lipopolysaccharide-stimulated mouse peritoneal macrophages and identification of active components. J. Appl. Phycol. 2017, 29, 563–573. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Naini, B.V.; Cortina, G. A histopathologic scoring system as a tool for standardized reporting of chronic (ileo) colitis and independent risk assessment for inflammatory bowel disease. Hum. Pathol. 2012, 43, 2187–2196. [Google Scholar] [CrossRef]
- Neri, B.; Mossa, M.; Scucchi, L.; Sena, G.; Palmieri, G.; Biancone, L. Histological scores in inflammatory bowel disease. J. Dig. Dis. 2021, 22, 9–22. [Google Scholar] [CrossRef]
- Medina, C.; Radomski, M.W. Role of matrix metalloproteinases in intestinal inflammation. J. Pharmacol. Exp. Ther. 2006, 318, 933–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, M.K.; Sakurai, H.; Kim, D.H.; Saiki, I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol. Rep. 2008, 19, 595–600. [Google Scholar] [PubMed]
- Huang, X.L.; Xu, J.; Zhang, X.H.; Qiu, B.Y.; Peng, L.; Zhang, M.; Gan, H.T. PI3K/Akt signaling pathway is involved in the pathogenesis of ulcerative colitis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2011, 60, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Godat, S.; Fournier, N.; Safroneeva, E.; Juillerat, P.; Nydegger, A.; Straumann, A.; Vavricka, S.; Biedermann, L.; Greuter, T.; Fraga, M.; et al. Frequency and type of drug-related side effects necessitating treatment discontinuation in the Swiss Inflammatory Bowel Disease Cohort. Eur. J. Gastroenterol. Hepatol. 2018, 30, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Ordas, I.; Cabre, E.; Garcia-Sanchez, V.; Bastida, G.; Penalva, M.; Gomollon, F.; Garcia-Planella, E.; Merino, O.; Gutierrez, A.; et al. Safety of thiopurine therapy in inflammatory bowel disease: Long-term follow-up study of 3931 patients. Inflamm. Bowel Dis. 2013, 19, 1404–1410. [Google Scholar] [CrossRef]
- Jeon, Y.; Jung, Y.; Kim, M.C.; Kwon, H.C.; Kang, K.S.; Kim, Y.K.; Kim, S.N. Sargahydroquinoic acid inhibits TNFalpha-induced AP-1 and NF-kappaB signaling in HaCaT cells through PPARalpha activation. Biochem. Biophys. Res. Commun. 2014, 450, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.J.; Han, S.C.; Yoon, W.J.; Koh, Y.S.; Hyun, J.W.; Kang, H.K.; Youl Cho, J.; Yoo, E.S. Sargaquinoic acid isolated from Sargassum siliquastrum inhibits lipopolysaccharide-induced nitric oxide production in macrophages via modulation of nuclear factor-kappaB and c-Jun N-terminal kinase pathways. Immunopharmacol. Immunotoxicol. 2013, 35, 80–87. [Google Scholar] [CrossRef]
- Yang, E.J.; Ham, Y.M.; Yang, K.W.; Lee, N.H.; Hyun, C.G. Sargachromenol from Sargassum micracanthum inhibits the lipopolysaccharide-induced production of inflammatory mediators in RAW 264.7 macrophages. Sci. World J. 2013, 2013, 712303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Ahmad, A.; Kumar, A.; Vyawahare, A.; Raza, S.S.; Khan, R. Lipid-based nanocarrier-mediated targeted delivery of celecoxib attenuate severity of ulcerative colitis. Mater. Sci. Eng. C Mater. Biol Appl 2020, 116, 111103. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.; Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Chami, B.; Martin, N.J.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch. Biochem. Biophys. 2018, 645, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.; Davies, M.J.C.m.c. Reactions of myeloperoxidase-derived oxidants with biological substrates: Gaining chemical insight into human inflammatory diseases. Curr. Med. Chem. 2006, 13, 3271–3290. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Han, G.C.; Wang, R.X.; Xiao, H.; Hou, C.M.; Guo, R.F.; Dou, Y.; Shen, B.F.; Li, Y.; et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal Immunol. 2014, 7, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.H.; Murakami, A.; Hayashi, R.; Ohigashi, H. Interleukin-1beta targets interleukin-6 in progressing dextran sulfate sodium-induced experimental colitis. Biochem. Biophys. Res. Commun. 2005, 337, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.M.; Thomas, A.G.; Akobeng, A.K. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Shibolet, O. Infliximab in ulcerative colitis. Biol. Targets Ther. 2008, 2, 379–388. [Google Scholar]
- Tountas, N.A.; Casini-Raggi, V.; Yang, H.; Di Giovine, F.S.; Vecchi, M.; Kam, L.; Melani, L.; Pizarro, T.T.; Rotter, J.I.; Cominelli, F. Functional and ethnic association of allele 2 of the interleukin-1 receptor antagonist gene in ulcerative colitis. Gastroenterology 1999, 117, 806–813. [Google Scholar] [CrossRef]
- Medina, C.; Videla, S.; Radomski, A.; Radomski, M.W.; Antolin, M.; Guarner, F.; Vilaseca, J.; Salas, A.; Malagelada, J.R. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G116–G122. [Google Scholar] [CrossRef]
- Castaneda, F.E.; Walia, B.; Vijay-Kumar, M.; Patel, N.R.; Roser, S.; Kolachala, V.L.; Rojas, M.; Wang, L.; Oprea, G.; Garg, P.; et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: Central role of epithelial-derived MMP. Gastroenterology 2005, 129, 1991–2008. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Takagi, T.; Kuroda, M.; Katada, K.; Ichikawa, H.; Kokura, S.; Yoshida, N.; Okanoue, T.; Yoshikawa, T. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium-induced colitis in mice. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2004, 53, 462–468. [Google Scholar] [CrossRef]
- O′Sullivan, S.; Gilmer, J.F.; Medina, C. Matrix metalloproteinases in inflammatory bowel disease: An update. Mediat. Inflamm. 2015, 2015, 964131. [Google Scholar] [CrossRef] [PubMed]
- Henninger, D.D.; Panes, J.; Eppihimer, M.; Russell, J.; Gerritsen, M.; Anderson, D.C.; Granger, D.N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J. Immunol. 1997, 158, 1825–1832. [Google Scholar]
- Hokari, R.; Kato, S.; Matsuzaki, K.; Kuroki, M.; Iwai, A.; Kawaguchi, A.; Nagao, S.; Miyahara, T.; Itoh, K.; Sekizuka, E.; et al. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to chronic colitis. Free. Radic. Biol. Med. 2001, 31, 153–163. [Google Scholar] [CrossRef]
- Wu, X.F.; Ouyang, Z.J.; Feng, L.L.; Chen, G.; Guo, W.J.; Shen, Y.; Wu, X.D.; Sun, Y.; Xu, Q. Suppression of NF-kappaB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol. Appl. Pharmacol. 2014, 281, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Karin, M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Jung, Y.C.; Jung, I.; Lee, H.W.; Youn, H.Y.; Lee, J.S. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C. Agardh on lipopolysaccharide-stimulated macrophage activation via NF-kappaB pathway regulation. Immunol. Investig. 2015, 44, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.H.; Kim, K.B.; Kim, M.J.; Kang, B.K.; Ahn, D.H. Anti-inflammatory activity of ethanolic extract of Sargassum micracanthum. J. Microbiol. Biotechnol. 2013, 23, 1691–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-W.; Ryu, B.-H.; Park, J.-W. Effects of sargassumpallidum on 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis in mice. J. Intern. Korean Med. 2010, 31, 224–241. [Google Scholar] [CrossRef]
- Gwon, W.G.; Lee, M.S.; Kim, J.S.; Kim, J.I.; Lim, C.W.; Kim, N.G.; Kim, H.R. Hexane fraction from Sargassum fulvellum inhibits lipopolysaccharide-induced inducible nitric oxide synthase expression in RAW 264.7 cells via NF-kappaB pathways. Am. J. Chin. Med. 2013, 41, 565–584. [Google Scholar] [CrossRef]
- Peng, X.D.; Wu, X.H.; Chen, L.J.; Wang, Z.L.; Hu, X.H.; Song, L.F.; He, C.M.; Luo, Y.F.; Chen, Z.Z.; Jin, K.; et al. Inhibition of phosphoinositide 3-kinase ameliorates dextran sodium sulfate-induced colitis in mice. J. Pharmacol. Exp. Ther. 2010, 332, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Cao, L.; Lee, S.G.; Park, S.-H.; Kim, H.-R. Sargahydroquinoic acid (SHQA) suppresses cellular senescence through Akt/mTOR signaling pathway. Exp. Gerontol. 2021, 151, 111406. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joung, E.-J.; Cao, L.; Gwon, W.-G.; Kwon, M.-S.; Lim, K.T.; Kim, H.-R. Meroterpenoid-Rich Ethanoic Extract of Sargassum macrocarpum Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice. Foods 2022, 11, 329. https://doi.org/10.3390/foods11030329
Joung E-J, Cao L, Gwon W-G, Kwon M-S, Lim KT, Kim H-R. Meroterpenoid-Rich Ethanoic Extract of Sargassum macrocarpum Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice. Foods. 2022; 11(3):329. https://doi.org/10.3390/foods11030329
Chicago/Turabian StyleJoung, Eun-Ji, Lei Cao, Wi-Gyeong Gwon, Mi-Sung Kwon, Kwon Taek Lim, and Hyeung-Rak Kim. 2022. "Meroterpenoid-Rich Ethanoic Extract of Sargassum macrocarpum Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice" Foods 11, no. 3: 329. https://doi.org/10.3390/foods11030329