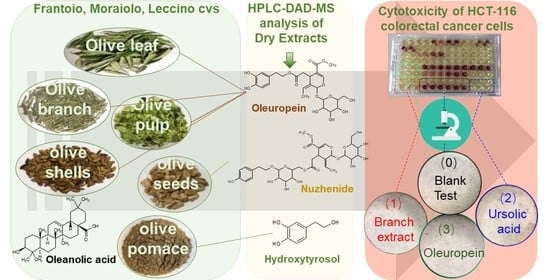
Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical Agents
2.3. Moisture Content
2.4. Extraction Protocols
2.5. Simultanous Measurement of Phenolic Compounds and Triterpenoids by HPLC-DAD-MS
2.6. HCT-116 Colon Cell Cytotoxicity Assessment with MTS Test
2.7. Data Analysis
3. Results and Discussion
3.1. Phenolic Compounds in the Extracts from Different Products of Frantoio, Leccino and Moraiolo Cultivars
3.2. Triterpenoids Level in Each Olive Extract for the Three Varieties
3.3. Cytotoxicity Evaluation of HCT-116 Human Colorectal Cell for the Olive Extracts from Frantoio
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Cavalheiro, C.V.; Picoloto, R.S.; Cichoski, A.J.; Wagner, R.; de Menezes, C.R.; Zepka, L.Q.; Da Croce, D.M.; Barin, J.S. Olive leaves offer more than phenolic compounds-fatty acids and mineral composition of varieties from Southern Brazil. Ind. Crop. Prod. 2015, 71, 122–127. [Google Scholar] [CrossRef]
- Fernández-Lobato, L.; López-Sánchez, Y.; Blejman, G.; Jurado, F.; Moyano-Fuentes, J.; Vera, D. Life cycle assessment of the Spanish virgin olive oil production: A case study for Andalusian region. J. Clean. Prod. 2021, 290, 125677. [Google Scholar] [CrossRef]
- Bartolini, G.; Prevost, G.; Messeri, C.; Carignani, G.; Menini, U. Olive Germplasm: Cultivars and World-Wide Collections; FAO: Rome, Italy, 1998. [Google Scholar]
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery based on olive biomass. State of the art and future trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Cecchi, L.; Innocenti, M.; Melani, F.; Migliorini, M.; Conte, L.; Mulinacci, N. New isobaric lignans from Refined Olive Oils as quality markers for Virgin Olive Oils. Food Chem. 2017, 219, 148–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinda, Á.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Romani, A.; Mulinacci, N.; Innocenti, M. Recovery and stability over time of phenolic fractions by an industrial filtration system of olive mill wastewater: A three-year study. J. Sci. Food Agric. 2018, 98, 2761–2769. [Google Scholar] [CrossRef]
- Bearth, A.; Cousin, M.E.; Siegrist, M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Qual. Prefer. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trend Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, R.H.; Wang, M.; Xu, G.B.; Liao, S.G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017, 131, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Bellumori, M.; De Marchi, L.; Mainente, F.; Zanoni, F.; Cecchi, L.; Innocenti, M.; Mulinacci, N.; Zoccatelli, G. A by-product from virgin olive oil production (pâté) encapsulated by fluid bed coating: Evaluation of the phenolic profile after shelf-life test and in vitro gastrointestinal. Int. J. Food Sci. Technol. 2021, 56, 3773–3783. [Google Scholar] [CrossRef]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G. Phenolic compounds of olive fruit: One- and two-dimensional nuclear magnetic resonance characterization of nuzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 1999, 47, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Rotelli, M.T.; Bocale, D.; De Fazio, M.; Ancona, P.; Scalera, I.; Memeo, R.; Travaglio, E.; Zbar, A.P.; Altomare, D.F. In-vitro evidence for the protective properties of the main components of the Mediterranean diet against colorectal cancer: A systematic review. Surg. Oncol. 2015, 24, 145–152. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, B.Y.; Li, P.; Wen, X.D.; Yang, J. Maslinic acid inhibits colon tumorigenesis by the AMPK-mTOR signaling pathway. J. Agric. Food Chem. 2019, 67, 4259–4272. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Japon-Lujan, R.; Priego Capote, F.; Luque de Castro, M.D. Temporal metabolomic analysis of o-glucoside phenolic compounds and their aglycone forms in olive tree and derived materials. Phytochem. Anal. 2008, 20, 221–230. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitao, F.; Bronze, M.; Coelho, A.V.; Vilas Boas, L. Secoiridoids in olive seed: Characterization of nüzhenide and 11-methyl oleosides by liquid chromatography with diode array and mass spectrometry. Grasas Y Aceites 2010, 61, 157–164. [Google Scholar] [CrossRef]
- Gomez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Del Carlo, M.; Compagnone, D.; Cichelli, A. Effects of fly attack (Bactrocera oleae) on the phenolic profile and selected chemical parameters of olive oil. J. Agric. Food Chem. 2008, 56, 4577–4583. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Zanoni, B.; Breschi, C.; Mulinacci, N. An effective HPLC-based approach for the evaluation of the content of total phenolic compounds transferred from olives to virgin olive oil during the olive milling process. J. Sci. Food Agric. 2018, 98, 3636–3643. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Romero, C.; Brenes, B. Bioactive substances in black ripe olives produced in Spain and the USA. J. Food Comp. Anal. 2018, 66, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Innocenti, M.; Mulinacci, N. Whole lyophilized olives as sources of unexpectedly high amounts of secoiridoids: The case of three Tuscan cultivars. J. Agric. Food Chem. 2015, 63, 1175–1185. [Google Scholar] [CrossRef]
- IOC. Determination of Biophenols in Olive Oils by HPLC; International Olive Council COI/T.20/Doc No. 29; IOC: Madrid, Spain, 2009. [Google Scholar]
- Tóth, G.; Alberti, Á.; Sólyomváry, A.; Barabás, C.; Boldizsár, I.; Noszál, B. Phenolic profiling of various olive bark-types and leaves: HPLC–ESI/MS study. Ind. Crop. Prod. 2015, 67, 432–438. [Google Scholar] [CrossRef]
- Obied, H.K.; Karuso, P.; Prenzler, P.D.; Robards, K. Novel secoiridoids with antioxidant activity from Australian olive mill waste. J. Agric. Food Chem. 2007, 55, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Guerrini, L.; Bellumori, M.; Balli, D.; Xie, P.; Parenti, A.; Mulinacci, N. Optimization of the production process of dried unripe olives (Olea europaea L.) as a nutraceutical ingredient naturally rich in phenolic compounds. LWT-Food Sci. Technol. 2020, 129, 109569. [Google Scholar] [CrossRef]
- Gutierrez-Rosales, F.; Romero, M.P.; Casanovas, M.; Motilva, M.J.; Mínguez-Mosquera, M.I. β-Glucosidase involvement in the formation and transformation of oleuropein during the growth and development of olive fruits (Olea europaea L. cv. Arbequina) grown under different farming practices. J. Agric. Food Chem. 2012, 60, 4348–4358. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A.; Montedoro, G. High-performance liquid chromatography evaluation of phenols in olive fruit, virgin olive oil, vegetation waters, and pomace and 1D- and 2D-nuclear magnetic resonance characterization. J. Am. Oil Chem. Soc. 1999, 76, 873–882. [Google Scholar] [CrossRef]
- Gutierrez-Rosales, F.; Romero, M.P.; Casanovas, M.; Motilva, M.J.; Minguez-Mosquera, M.I. Metabolites involved in oleuropein accumulation and degradation in fruits of Olea Europaea L., Hojiblanca and Arbequina varieties. J. Agric. Food Chem. 2010, 58, 12924–12933. [Google Scholar] [CrossRef] [PubMed]
- Rovellini, P.; Cortesi, N. Liquid chromatography-mass spectrometry in the study of oleuropein and ligstroside aglycons in virgin olive oil: Aldehydic, dialdehydic forms and their oxidized products. Riv. Ital. Delle Sostanze Grasse 2002, 79, 1–14. [Google Scholar]
- Briante, R.; Patumi, M.; Limongelli, S.; Febbraio, F.; Vaccaro, C.; Di Salle, A.; La Cara, F.; Nucci, R. Changes in phenolic and enzymatic activities content during fruit ripening in two Italian cultivars of Olea europea L. Plant Sci. 2002, 162, 791–798. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Uceda, M.; Paz Aguilera, M.; Gaforio, J.J.; Beltrán, G. Influence of olive paste preparation conditions on virgin olive oil triterpenic compounds at laboratory-scale. Food Chem. 2010, 119, 765–769. [Google Scholar] [CrossRef]
- Vukovic, N.L.; Obradovic, A.D.; Vukic, M.D.; Jovanovic, D.; Djurdjevic, P.M. Cytotoxic, proapoptotic and antioxidative potential of flavonoids isolated from propolis against colon (HCT-116) and breast (MDA-MB-231) cancer cell lines. Food Res. Int. 2018, 106, 71–80. [Google Scholar] [CrossRef]
- Fabiani, R.; Morozzi, G. Chapter 105—Anticarcinogenic properties of olive oil phenols: Effects on proliferation, apoptosis and differentiation. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 981–988. [Google Scholar]
- Shi, J.; Shan, S.; Li, Z.; Li, H.; Li, X.; Li, Z. Bound polyphenol from foxtail millet bran induces apoptosis in HCT-116 cell through ROS generation. J. Funct. Foods 2015, 17, 958–968. [Google Scholar] [CrossRef]
- Kampa, M.; Nifli, A.P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 2007, 159, 79–113. [Google Scholar] [PubMed]
- Magne Nde, C.B.; Zingue, S.; Winter, E.; Creczynski-Pasa, T.B.; Michel, T.; Fernandez, X.; Njamen, D.; Clyne, C. Flavonoids, breast cancer chemopreventive and/or chemotherapeutic agents. Curr. Med. Chem. 2015, 22, 3434–3446. [Google Scholar]
- Hamdi, H.K.; Castellon, R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Comm. 2005, 334, 769–778. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef] [PubMed]







| Oil and By-Products | Frantoio | Leccino | Moraiolo | Treatment Ways |
|---|---|---|---|---|
| Extra virgin olive oil | 500 mL, light green | 500 mL, yellowish | 500 mL, light green | Directly obtained from olive mill for use |
| Olive leaf | 6~7 × 1.4~1.5 cm | 6~7 × 1.4~1.5 cm | 5~6 × 1.4~1.5 cm | Naturally dried indoor at R.T. for 5 days, and pulverization |
| Olive pomace (none stone) | Gray-yellow powder after being lyophilized | Gray-yellow powder after being lyophilized | Gray-yellow powder after being lyophilized | Lyophilized for 2 days and then remove stone and pulverization |
| Olive fruit (none stone) | 1.7~1.8 cm × 1.3~1.4 cm green & dark | 1.8~1.9 × 1.3~1.4 cm mostly dark | 1.7~1.8 × 1.2~1.3 cm green & dark | Lyophilized for 2 days and then destoned with manual handing and pulverization |
| Olive shell (none seed) | 1.1~1.2 × 0.6~0.7 cm | 1.3~1.5 × 0.65~0.7 cm | 1.15~1.2 × 0.65~0.7 cm | Naturally dried indoor at R.T. for 5 days, and then separate seed cautiously with a hammer and pulverization |
| Olive seed | 0.9~1.0 × 0.4~0.45 cm | 1.0~1.1 × 0.4~0.5 cm | 0.85~0.95 × 0.35~0.4 cm | The intact seed was kept in darkness and naturally dried for 3 days and pulverization |
| Olive branch | 45~50 cm cut from the top with cut diameter: 3~5 mm containing 0.3~0.4 mm thickness of the peel | 45~50 cm cut from the top with cut diameter: 3~5 mm containing 0.3~0.4 mm thickness of the peel | 45~50 cm cut from the top with cut diameter: 3~5 mm containing 0.3~0.4 mm thickness of the peel | Naturally dried indoor at R.T. for 5 days, and pulverization |
| mg/kg | ||||||||
|---|---|---|---|---|---|---|---|---|
| Triterpenoid. (rt, min) | cv | Fruits | Leaves | Pomace | Branches | Shell | Seeds | Oil |
| Maslinic acid (49.5) | Frantoio | 2277 c | 2434 b | 1319 b | 1745 a | nd | nd | 82.55 b |
| Leccino | 4003 b | 2583 b | 3442 a | 3685 a | nd | nd | 126.40 a | |
| Moraiolo | 5805 a | 4534 a | 551 c | 5916 a | nd | nd | 36.97 c | |
| Oleanolic acid (53.2) | Frantoio | 1142 b | 17036 a | 783 a | 6027 b | nd | nd | 25.75 a |
| Leccino | 1925 a | 11880 b | 940 a | 10104 a | nd | nd | 37.70 a | |
| Moraiolo | 2265 a | 13121 b | 367 b | 11374 a | nd | nd | 40.20 a | |
| Ursolic acid (53.3) | Frantoio | nd | nd | nd | 5386 a | nd | nd | 46.01 b |
| Leccino | nd | 5556 a | 738 | 5321 a | nd | 7338 | 92.63 a | |
| Moraiolo | nd | 6334 a | nd | 7605 a | nd | nd | 46.80 b | |
| Erythrodiol (55.4) | Frantoio | nd | 3219 a | nd | 976 b | nd | nd | 51.00 b |
| Leccino | nd | nd | nd | 2148 a | nd | 2717 | 16.97 c | |
| Moraiolo | nd | 1358 b | nd | nd | nd | nd | 169.53 a | |
| Total Triterpenoids level | Frantoio | 3419 c | 22689 b | 2102 b | 14134 b | nd | nd | 205.3 b |
| Leccino | 5928 b | 20019 c | 5120 a | 21258 a | nd | 10055 | 273.7 a | |
| Moraiolo | 8115 a | 25347 a | 918 c | 24895 a | nd | nd | 293.5 a | |
| A | Leaf | Branch | Fruit | Seeds | Pomace | Shell | Oil |
|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | 97.06 | 88.25 | 154.3 | 875.5 | 95.85 | 140.5 | 170.0 |
| B | OH-Tyrosol | Verbascoside | Clorogenic acid | Caffeic acid | Oleuropein | ||
| IC50 (µM) | 66 | 79 | 207 | 145 | 61 | ||
| Rutin | Luteolin 7-O-glucoside | Quercetin | Luteolin | Taxifolin | |||
| IC50 (µM) | 940 | 58 | 38 | 89 | 200 | ||
| Maslinic acid | Ursolic Acid | Erythrodiol | |||||
| IC50 (µM) | 41 | 24 | 69 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods 2021, 10, 2823. https://doi.org/10.3390/foods10112823
Xie P, Cecchi L, Bellumori M, Balli D, Giovannelli L, Huang L, Mulinacci N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods. 2021; 10(11):2823. https://doi.org/10.3390/foods10112823
Chicago/Turabian StyleXie, Pujun, Lorenzo Cecchi, Maria Bellumori, Diletta Balli, Lisa Giovannelli, Lixin Huang, and Nadia Mulinacci. 2021. "Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.)" Foods 10, no. 11: 2823. https://doi.org/10.3390/foods10112823