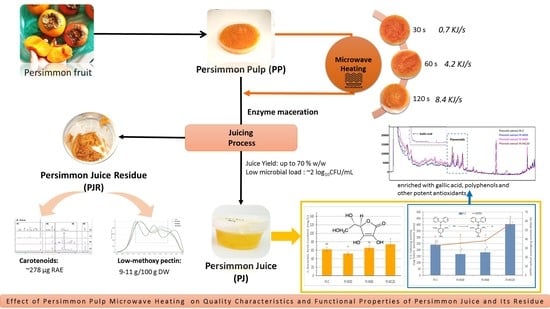
On the Effect of Microwave Heating on Quality Characteristics and Functional Properties of Persimmon Juice and Its Residue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Material and Sample Preparation
2.3. Microwave Heating
2.4. Persimmon Juice Preparation
2.5. Tests on Persimmon Juice
2.5.1. Microbiological Analysis
2.5.2. Total Soluble Solids and Individual Sugars
2.5.3. Total Acidity, pH, and Organic Acids’ Composition
2.5.4. Color Evaluation
2.5.5. L-Ascorbic Acid Determination
2.5.6. Preparation of Phenolic-Rich Extracts
2.5.7. Folin–Ciocalteu (F–C) Assay
2.5.8. RP-UHPLC-DAD-FLD Analysis of Phenolic Compounds
2.5.9. DPPH● Scavenging Assay
2.6. Tests on Persimmon Juice Residue
2.6.1. Extraction and Purification of Pectin
2.6.2. Preparation of Carotenoid-Rich Extracts
2.6.3. Total Carotenoid Content Estimation
2.6.4. Carotenoid Profiling by HPLC‒DAD
2.7. Statistical Analysis
3. Results and Discussion
3.1. Persimmon Juice
3.1.1. Juice Yield and Physicochemical Characteristics
3.1.2. Microbiological Quality and Safety
3.1.3. L-Ascorbic Acid Content
3.1.4. Antioxidant Potential
3.2. Persimmon Juice Residue
3.2.1. Pectin Content and Structural Features
3.2.2. Total and Individual Carotenoid Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMCs | Aerobic mesophilic counts |
| DPPH● | 1,1-diphenyl-2-picrylhydrazyl radical |
| F-C | Folin–Ciocalteu |
| GAE | Gallic acid equivalents |
| MW | Microwave |
| PJ | Persimmon juice |
| PJR | Persimmon juice residue |
| PP | Persimmon pulp |
| RAE | Retinol activity equivalents |
| TA | Titratable acidity |
| YMCs | Yeasts and molds counts |
| β-Car | β-carotene |
| β-Cry | β-cryptoxanthin |
Appendix A
| No | Peak Elution Time Interval, ΔtR (min) 1 | λmax (nm) | Possible Identity |
|---|---|---|---|
| 1 | 5.14–5.24 | 407 | FX, ni 2 |
| 2 | 5.48–5.55 | 426 | FX, ni |
| 3 | 5.99–6.16 | (328),415,440,470 | FX, ni |
| 4 | 6.30–6.45 | (389), 412,426,450 | (all-E)-Luteoxanthin |
| 5 | 6.78–6.92 | 415,439,469 | (all-E)-Violaxanthin |
| 6 | 7.09–7.28 | 414,432,464 | FX, ni |
| 7 | 7.85–8.08 | (340),414,439,469 | FX, ni |
| 8 | 8.11–8.35 | 404,422,450 | FX, ni |
| 9 | 8.53–8.68 | 410,436,467 | FX, ni |
| 10 | 9.20–9.45 | 415,444,472 | (all-E)-Antheraxanthin |
| 11 | 9.73–9.93 | 398,419,442 | FX, ni |
| 12 | 10.05–10.18 | 397,420,442 | FX, ni |
| 13 | 10.57–10.72 | 432,451 | FX, ni |
| 14 | 11.18–11.45 | 422,448,473 | (all-E)-Lutein |
| 15 | 11.68–11.91 | 426 | FX, ni |
| 16 | 12.49–12.83 | 423,451,477 | (all-E)-Zeaxanthin |
| 17 | 13.30–13.69 | 420, 442, 469 | 5,6-epoxy- Lutein |
| 18 | 14.01–14.34 | 429,453 | FX, ni |
| 19 | 14.34–14.61 | 443,473 | FX, ni |
| 20 | 14.85–15.09 | 418,445,473 | FX, ni |
| 21 | 15.16–15.28 | 410,443,470 | FX, ni |
| 22 | 15.41–16.00 | 335,423,450,467 | FX, ni |
| 23 | 15.93–16.13 | 335,423,449,467 | FX, ni |
| 24 | 15.98–16.33 | 334,442,469 | Unknown+5,6-epoxy- β-Cryptoxanthin |
| 25 | 16.74–17.05 | 450 | FX, ni |
| 26 | 17.16–17.93 | 440 | FX, ni |
| 27 | 17.50–17.88 | 440 | FX, ni |
| 28 | 18.34–18.64 | 421,446 | FX, ni |
| 29 | 18.81–19.06 | (428),451,478 | (all-E)-β-Cryptoxanthin |
| 30 | 19.85–20.39 | 440 | FX, ni |
| 31 | 20.55–21.05 | 450,475 | ni |
| 32 | 21.51–22.03 | (327),446,473 | ni |
| 33 | 22.19–22.60 | 450 | ni |
| 34 | 22.72–22.95 | (359),379,399,423 | Luteoxanthin or Neoxanthin ester |
| 35 | 23.70–23.93 | 335,421,449,473 | HC, ni |
| 36 | 24.28–24.71 | (330),421,446,472 | ni |
| 37 | 24.85–25.23 | 454,470 | ni |
| 38 | 25.01–25.47 | 450 | ni |
| 39 | 25.89–26.19 | 426,452,478 | (all-E)-β-carotene |
| 40 | 26.51–27.19 | 419,438,469 | violaxanthin ester |
| 41 | 26.98–27.81 | (322),420,445,470 | (9Z)-β-carotene |
| 42 | 27.61–28.11 | 418,441,469 | XE, ni |
| 43 | 27.89–28.87 | 425,441,473 | XE, ni |
| 44, 45 | 28.96–29.82 | 329,434,465 | XE, ni |
| 46, 47 | 29.75–30.74 | (394),418,441,468 | XE, ni |
| 48 | 31.08–32.06 | (329),412,438,465 | XE, ni |
| 49 | 31.82–32.21 | 420,447,469 | (all-E)-zeaxanthin palmitate |
| 50 | 32.33–33.40 | 426,450,477 | (all-E)-β-cryptoxanthin laurate |
| 51 | 32.94–34.20 | 424,446,473 | (all-E)-lutein 3-O-palmitate |
| 52 | 33.70–34.60 | (311),418,445,469 | XE, ni |
| 53 | 35.09–35.59 | 424,449,473 | (all-E)-zeaxanthin myristate |
| 54 | 35.36–35.99 | (334),450,477 | XE, ni |
| 55 | 35.85–36.33 | 420,446,473 | (all-E)-antheraxanthin ester |
| 56 | 36.07–36.96 | 451,478 | (all-E)-β-cryptoxanthin myristate |
| 57 | 37.34–38.38 | (425),451,478 | XE, ni |
| 58 | 38.12–38.63 | 452,474 | XE, ni |
| 59 | 38.94–39.49 | 451,475 | XE, ni |
| 60 | 39.53–40.17 | 450,469 | XE, ni |
| 61 | 40.14–40.98 | 450,469 | XE, ni |
| 62 | 45.33–46.12 | 446,472,503 | lycopene |
References
- Llácer, G.; Badenes, M.L. Persimmon production and market. Options Mediterr. 2002, 51, 9–21. [Google Scholar]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros kaki) fruit: Hidden phytochemicals and health claims. EXCLI J. 2015, 14, 542–561. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Corporate Statistical Database (FAOSTAT) Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 September 2021).
- Pastopoulos, S.; Kazantzis, K.; Marsanidis, S. The cultivation of Persimmon in Yianitsa region (in Greek). Agrotypos 2017, 10, 41–47. [Google Scholar]
- Ordoudi, S.A.; Bakirtzi, C.; Tsimidou, M.Z. The potential of tree fruit stone and seed wastes in Greece as sources of bioactive ingredients. Recycling 2018, 3, 9. [Google Scholar] [CrossRef] [Green Version]
- Curi, P.N.; Tavares, B.S.; Almeida, A.B.; Pio, R.; Pasqual, M.; Peche, P.M.; de Souza, V.R. Characterization and influence of subtropical persimmon cultivars on juice and jelly characteristics. An. Acad. Bras. Cienc. 2017, 89, 1205–1220. [Google Scholar] [CrossRef] [Green Version]
- González, E.; Vegara, S.; Martí, N.; Valero, M.; Saura, D. Physicochemical characterization of pure persimmon juice: Nutritional quality and food acceptability. J. Food Sci. 2015, 80, C532–C539. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Marti, N.; Saura, D.; Valero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Characterization of polyphenols, sugars, and other polar compounds in persimmon juices produced under different technologies and their assessment in terms of compositional variations. Food Chem. 2015, 182, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Gea-Botella, S.; Agulló, L.; Martí, N.; Martínez-Madrid, M.C.; Lizama, V.; Martín-Bermudo, F.; Berná, G.; Saura, D.; Valero, M. Carotenoids from persimmon juice processing. Food Res. Int. 2021, 141. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 2: Effect on composition, phytochemical content, and physicochemical, rheological, and organoleptic properties of fruit juices. Crit. Rev. Food Sci. Nutr. 2017, 57, 637–652. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef]
- Markets and Markets. Carotenoids Market by Type, Application, Region—Global Forecast 2026|COVID-19 Impact on Carotenoids Market; Markets and Markets: Seattle, WA, USA, 2019. [Google Scholar]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; UN General Assembly: New York, NY, USA, 2015; pp. 1–35. [Google Scholar]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin extracted from persimmon peel: A physicochemical characterization and emulsifying properties evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 501–523. [Google Scholar] [CrossRef]
- Gerard, K.A.; Roberts, J.S. Microwave heating of apple mash to improve juice yield and quality. LWT-Food Sci. Technol. 2004, 37, 551–557. [Google Scholar] [CrossRef]
- Cendres, A.; Chemat, F.; Page, D.; Le Bourvellec, C.; Markowski, J.; Zbrzezniak, M.; Renard, C.M.G.C.; Plocharski, W. Comparison between microwave hydrodiffusion and pressing for plum juice extraction. LWT-Food Sci. Technol. 2012, 49, 229–237. [Google Scholar] [CrossRef]
- Pérez-Grijalva, B.; Herrera-Sotero, M.; Mora-Escobedo, R.; Zebadúa-García, J.C.; Silva-Hernández, E.; Oliart-Ros, R.; Pérez-Cruz, C.; Guzmán-Gerónimo, R. Effect of microwaves and ultrasound on bioactive compounds and microbiological quality of blackberry juice. LWT-Food Sci. Technol. 2018, 87, 47–53. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Papapostolou, M.; Mantzouridou, F.T.; Tsimidou, M.Z. Flavored olive oil as a preservation means of reduced salt spanish style green table olives (Cv. chalkidiki). Foods 2021, 10, 392. [Google Scholar] [CrossRef]
- Lalou, S.; Hatzidimitriou, E.; Papadopoulou, M.; Kontogianni, V.G.; Tsiafoulis, C.G.; Gerothanassis, I.P.; Tsimidou, M.Z. Beyond traditional balsamic vinegar: Compositional and sensorial characteristics of industrial balsamic vinegars and regulatory requirements. J. Food Compos. Anal. 2015, 43, 175–184. [Google Scholar] [CrossRef]
- Horwitz, W. (Ed.) Official Methods of Analysis of Association of Official Analytical Chemists International Method No. 982.27; AOAC Press: Arlington, VA, USA, 2003. [Google Scholar]
- Ordoudi, S.A.; Mantzouridou, F.; Daftsiou, E.; Malo, C.; Hatzidimitriou, E.; Nenadis, N.; Tsimidou, M.Z. Pomegranate juice functional constituents after alcoholic and acetic acid fermentation. J. Funct. Foods 2014, 8, 161–168. [Google Scholar] [CrossRef]
- Cheng, C.X.; Jia, M.; Gui, Y.; Ma, Y. Comparison of the effects of novel processing technologies and conventional thermal pasteurisation on the nutritional quality and aroma of Mandarin (Citrus unshiu) juice. Innov. Food Sci. Emerg. Technol. 2020, 64, 102425. [Google Scholar] [CrossRef]
- AOAC. AOAC official method 967.21 Ascorbic Acid in Vitaimin Prepaprations and juices 2,6-Dichloroindophenol titremetric method. In Official Methods of Analysis; AOAC Press: Arlington, VA, USA, 2006. [Google Scholar]
- Ubeda, C.; Callejón, R.M.; Hidalgo, C.; Torija, M.J.; Mas, A.; Troncoso, A.M.; Morales, M.L. Determination of major volatile compounds during the production of fruit vinegars by static headspace gas chromatography-mass spectrometry method. Food Res. Int. 2011, 44, 259–268. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Sotiroglou, M.; Mastralexi, A.; Nenadis, N.; García-González, D.L.; Toschi, T.G. In house validated UHPLC protocol for the determination of the total hydroxytyrosol and tyrosol content in virgin olive oil fit for the purpose of the health claim introduced by the EC Regulation 432/2012 for “olive oil polyphenols”. Molecules 2019, 24, 1044. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.L.; Lin, J.T.; Lin, H.L.; Liao, P.L.; Wu, P.J.; Yang, D.J. Phenolic compositions and antioxidant properties of leaves of eight persimmon varieties harvested in different periods. Food Chem. 2019, 289, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Caballero, M.P.; Acedo-Valenzuela, M.I.; Galeano-Díaz, T. Simple quantification of phenolic compounds present in the minor fraction of virgin olive oil by LC-DAD-FLD. Talanta 2012, 101, 479–487. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Giuffrida, D.; Mangraviti, D.; Rigano, F.; Mondello, L.; Arlorio, M.; Coïsson, J.D. Characterization of peel and pulp proanthocyanidins and carotenoids during ripening in persimmon “Kaki Tipo” cv, cultivated in Italy. Food Res. Int. 2019, 120, 800–809. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; Fernández-López, R.; Welti-Chanes, J.; García-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; Welti-Chanes, J.; García-Cayuela, T. Characterization of carotenoid and carotenoid esters of astringent persimmon tissues (Diospyros kaki Thunb. var. Rojo brillante). Effects of thermal and high pressure non-thermal processing M. Preprints 2018. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Blanco-Navarro, I.; Pérez-Sacristán, B.; Blázquez-García, S. In vitro bioaccessibility of carotenoids and tocopherols from fruits and vegetables. Food Chem. 2007, 102, 641–648. [Google Scholar] [CrossRef]
- Veberic, R.; Jurhar, J.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.). Food Chem. 2010, 119, 477–483. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.B.; Seo, W.D.; Kang, S.T.; Lim, J.W.; Cho, K.M. Comparative studies of antioxidant activities and nutritional constituents of persimmon juice (Diospyros kaki L. cv. Gapjubaekmok). Prev. Nutr. Food Sci. 2012, 17, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Koskiniemi, C.B.; Truong, V.D.; McFeeters, R.F.; Simunovic, J. Quality evaluation of packaged acidified vegetables subjected to continuous microwave pasteurization. LWT-Food Sci. Technol. 2013, 54, 157–164. [Google Scholar] [CrossRef]
- Cendres, A.; Chemat, F.; Maingonnat, J.F.; Renard, C.M.G.C. An innovative process for extraction of fruit juice using microwave heating. LWT-Food Sci. Technol. 2011, 44, 1035–1041. [Google Scholar] [CrossRef]
- Stinco, C.M.; Szczepańska, J.; Marszałek, K.; Pinto, C.A.; Inácio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Meléndez-Martínez, A.J. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef] [PubMed]
- European Commission. COMMISSION REGULATION (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 51, 141–166. [Google Scholar]
- Mendes-Oliveira, G.; Deering, A.J.; San Martin-Gonzalez, M.F.; Campanella, O.H. Microwave pasteurization of apple juice: Modeling the inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium at 80–90 °C. Food Microbiol. 2020, 87, 103382. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R. Effects of Microwave Pretreatment of Apple Raw Material on the Nutrients and Antioxidant Activities of Apple Juice. J. Food Process. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dars, A.G.; Hu, K.; Liu, Q.; Abbas, A.; Xie, B.; Sun, Z. Effect of thermo-sonication and ultra-high pressure on the quality and phenolic profile of mango juice. Foods 2019, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Lee, S.C. Effect of heat treatment condition on the antioxidant and several physiological activities of non-astringent persimmon fruit juice. Food Sci. Biotechnol. 2012, 21, 815–822. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; Pereira, L.J.; Gut, J.A.W. Inactivation Kinetics of Pectin Methylesterase, Polyphenol Oxidase, and Peroxidase in Cloudy Apple Juice under Microwave and Conventional Heating to Evaluate Non-Thermal Microwave Effects. Food Bioprocess Technol. 2018, 11, 1359–1369. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V. A Review -Pectin from Agro and Industrial Waste. Int. J. Appl. Environ. Sci. ISSN 2017, 12, 973–6077. [Google Scholar]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit- and vegetable-based matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Hitaka, Y.; Nakano, A.; Tsukigawa, K.; Manabe, H.; Nakamura, H.; Nakano, D.; Kinjo, J.; Nohara, T.; Maeda, H. Characterization of carotenoid fatty acid esters from the peels of the persimmon Diospyros kaki. Chem. Pharm. Bull. 2013, 61, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Marangoni, A.G. Carotenoid Esters in Foods Food Chemistry, Function and Analysis; Mercadante, A.Z., Ed.; The Royal Society of Chemistry: London, UK, 2019; ISBN 9781788015851. [Google Scholar]
- Zhou, C.; Zhao, D.; Sheng, Y.; Tao, J.; Yang, Y. Carotenoids in fruits of different persimmon cultivars. Molecules 2011, 16, 624–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordani, E.; Doumett, S.; Nin, S.; Del Bubba, M. Selected primary and secondary metabolites in fresh persimmon (Diospyros kaki Thunb.): A review of analytical methods and current knowledge of fruit composition and health benefits. Food Res. Int. 2011, 44, 1752–1767. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]







| PJ-C | PJ-M30 | PJ-M60 | PJ-M120 | |
|---|---|---|---|---|
| Yield (% w/w) | 36.6 ± 1.5 a | 72.1 ± 4.8 b,c | 67.0 ± 3.7 b,c | 71.8 ± 1.5 b,c |
| Total soluble solids (°Brix) | 15.50 ± 0.32 b | 13.42 ± 0.59 a | 15.17 ± 0.82 b | 15.17 ± 0.26 b |
| Sugars | ||||
| Glucose (g/L) | 74.43 ± 7.28 a | 65.65 ± 6.40 a | 66.25 ± 6.32 a | 80.30 ± 1.10 a |
| Fructose (g/L) | 78.24 ± 8.37 a | 80.52 ± 4.49 a | 82.58 ± 4.49 a | 91.12 ± 1.16 a |
| Glucose-to-Fructose Ratio | 0.95 ± 0.01 c | 0.81 ± 0.03 a | 0.80 ± 0.03 a | 0.88 ± 0.01 b |
| pH | 3.12 ± 0.01 a | 2.91 ± 1.23 a | 3.32 ± 0.06 a | 3.40 ± 0.03 a |
| Total Acidity (g malic acid/100 mL) | 0.57 ± 0.03 a | 0.69 ± 0.04 b | 0.67 ± 0.04 b | 0.71 ± 0.03 b |
| Organic Acids | ||||
| Acetic acid (g/L) | 6.04 ± 0.15 a | 6.60 ± 0.12 b | 8.33 ± 0.25 c | 8.62 ± 0.27 c |
| Citric acid (g/L) | 1.19 ± 0.01 a | 1.80 ± 0.02 d | 1.59 ± 0.02 c | 1.49 ± 0.02 b |
| Tartaric acid (g/L) | 2.25 ± 0.03 b | 3.59 ± 0.05 c | 2.29 ± 0.04 b | 0.50 ± 0.01 a |
| Malic acid (g/L) | 1.84 ± 0.02 b | 2.86 ±0.03 c | 1.88 ± 0.02 b | 0.49 ± 0.01 a |
| L | 55.61 ± 0.01 a | 57.90 ± 0.31 b | 58.78 ± 0.71 c | 61.70 ± 0.14 d |
| a* | 2.59 ± 0.06 c | −0.16 ± 0.08 b | −0.24 ± 0.06 b | −1.69 ± 0.05 a |
| b* | 20.44 ± 8.61 b | 18.47 ± 1.27 b | 18.01 ± 0.53 b | 12.19 ± 0.14 a |
| C* | 20.72 ± 8.24 b,c | 18.47 ± 1.27 b | 18.01 ± 0.53 b | 12.30 ± 0.14 a |
| h | 77.80 ± 14.80 a | 90.49 ± 0.21 b | 90.77 ± 0.21 b | 97.88 ± 0.22 c |
| ΔE* | 0 | 4.08 ± 0.57 a | 4.90 ± 0.26 a | 11.11 ± 0.41 b |
| Sample | Pectin Content (g/100 g DW) |
| PP | 12.0 ± 0.9 c |
| PJR-C | 11.0 ± 0.7 b,c |
| PJR-M30 | 10.0 ± 0.9 a,b |
| PJR-M60 | 9.0 ± 0.9 a |
| PJR-M120 | 9.0 ± 0.9 a |
| PP | PJR-C | PJR-M30 | PJR-M60 | PJR-M120 | |
|---|---|---|---|---|---|
| Carotenoid content [% of total peak area at 450 nm] | |||||
| FX | 13.4 ± 2.5 | 9.2 ± 1.4 | 10.8 ± 1.4 | 51.9 ± 2.9 | 55.9 ± 0.5 |
| XE | 53.7 ± 2.7 | 57.6 ± 2.6 | 61.2 ± 3.2 | 15.8 ± 2.0 | 14.2 ± 0.3 |
| HC | 27.8 ± 1.4 | 27.6 ± 2.4 | 22.4 ± 0.4 | 22.1 ± 1.1 | 22.3 ± 0.2 |
| u | 5.5 ± 1.3 | 4.8 ± 1.1 | 5.2 ± 2.4 | 7.7 ± 1.4 | 7.7 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalou, S.; Ordoudi, S.A.; Mantzouridou, F.T. On the Effect of Microwave Heating on Quality Characteristics and Functional Properties of Persimmon Juice and Its Residue. Foods 2021, 10, 2650. https://doi.org/10.3390/foods10112650
Lalou S, Ordoudi SA, Mantzouridou FT. On the Effect of Microwave Heating on Quality Characteristics and Functional Properties of Persimmon Juice and Its Residue. Foods. 2021; 10(11):2650. https://doi.org/10.3390/foods10112650
Chicago/Turabian StyleLalou, Sofia, Stella A. Ordoudi, and Fani Th. Mantzouridou. 2021. "On the Effect of Microwave Heating on Quality Characteristics and Functional Properties of Persimmon Juice and Its Residue" Foods 10, no. 11: 2650. https://doi.org/10.3390/foods10112650