Influence of pH, Temperature and Protease Inhibitors on Kinetics and Mechanism of Thermally Induced Aggregation of Potato Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Modulated Differential Scanning Calorimetry (mDSC)
2.3. Heating Experiments
2.4. Size-Exclusion Chromatography Coupled with Fluorescence Intensity Detection
2.5. Determination of Free Thiol Groups in Heated PPI Solutions
2.6. Blocking of Protein Interactions
2.7. Gel Electrophoretic Analysis
2.8. Determination of Exposed Hydrophobicity
2.9. Data Evaluation and Kinetic Data Fit
3. Results and Discussion
3.1. Heat-Induced Denaturation and Aggregation in PPI Solutions
3.2. Kinetic Parameters of Patatin Denaturation and Aggregation
3.3. Protein–Protein Interactions within PPI Aggregates Measured by SDS-PAGE
3.4. Reduction of Free Thiol Groups in PPI Solutions and Formation of Disufide Bonds during Heat-Induced Aggregation
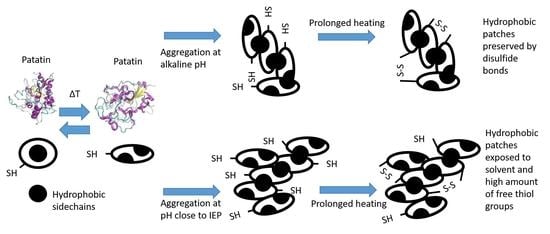
3.5. Change in the Hydrophobic Character of PPI Aggregates during Heat-Induced Aggregation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolai, T.; Durand, D. Controlled food protein aggregation for new functionality. Curr. Opin. Colloid Interface Sci. 2013, 18, 249–256. [Google Scholar] [CrossRef]
- Monahan, F.J.; German, J.B.; Kinsella, J.E. Effect of pH and temperature on protein unfolding and thiol/disulfide interchange reactions during heat-induced gelation of whey proteins. J. Agric. Food. Chem. 1995, 43, 46–52. [Google Scholar] [CrossRef]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Pots, A.M.; Gruppen, H.; Hessing, M.; van Boekel, M.A.; Voragen, A.G. Isolation and characterization of patatin isoforms. J. Agric. Food. Chem. 1999, 47, 4587–4592. [Google Scholar] [CrossRef]
- Racusen, D.; Foote, M. A major soluble Glycoprotein of potato tubers. J. Food Biochem. 1980, 4, 43–52. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L. Advances in Potato Chemistry and Technology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2016; ISBN 9780128000021. [Google Scholar]
- Pots, A.M.; Jongh, H.H.J.; Gruppen, H.; Hamer, R.J.; Voragen, A.G.J. Heat-induced conformational changes of patatin, the major potato tuber protein. Eur. J. Biochem. 1998, 252, 66–72. [Google Scholar] [CrossRef]
- Pots, A.M.; Gruppen, H.; Jongh, H.H.J.; van Boekel, M.A.J.S.; Walstra, P.; Voragen, A.G.J. Kinetic Modeling of the Thermal Aggregation of Patatin. J. Agric. Food. Chem. 1999, 47, 4593–4599. [Google Scholar] [CrossRef]
- Pots, A.M.; ten Grotenhuis, E.; Gruppen, H.; Voragen, A.G.J.; Kruif, K.G. Thermal Aggregation of Patatin Studied in Situ. J. Agric. Food. Chem. 1999, 47, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Delahaije, R.J.B.M.; Wierenga, P.A.; Giuseppin, M.L.F.; Gruppen, H. Comparison of heat-induced aggregation of globular proteins. J. Agric. Food. Chem. 2015, 63, 5257–5265. [Google Scholar] [CrossRef]
- Creusot, N.; Wierenga, P.A.; Laus, M.C.; Giuseppin, M.L.F.; Gruppen, H. Rheological properties of patatin gels compared with β-lactoglobulin, ovalbumin, and glycinin. J. Sci. Food Agric. 2011, 91, 253–261. [Google Scholar] [CrossRef]
- Pouvreau, L.; Gruppen, H.; Piersma, S.R.; van den Broek, L.A.M.; van Koningsveld, G.A.; Voragen, A.G.J. Relative Abundance and Inhibitory Distribution of Protease Inhibitors in Potato Juice from cv. Elkana. J. Agric. Food. Chem. 2001, 49, 2864–2874. [Google Scholar] [CrossRef]
- Løkra, S.; Helland, M.H.; Claussen, I.C.; Egelandsdal, B.; Strætkvern, K.O. Chemical characterization and functional properties of a potato protein concentrate prepared by large-scale expanded bed adsorption chromatography. LWT Food Sci. Technol. 2008, 41, 1089–1099. [Google Scholar] [CrossRef]
- Pouvreau, L.; Gruppen, H.; van Koningsveld, G.; van den Broek, L.A.M.; Voragen, A.G.J. Conformational stability of the potato serine protease inhibitor group. J. Agric. Food. Chem. 2005, 53, 3191–3196. [Google Scholar] [CrossRef]
- Pouvreau, L.; Kroef, T.; Gruppen, H.; van Koningsveld, G.; van den Broek, L.A.M.; Voragen, A.G.J. Structure and stability of the potato cysteine protease inhibitor group (cv. Elkana). J. Agric. Food. Chem. 2005, 53, 5739–5746. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Damgaard, H.; Greve-Poulsen, M.; Sunds, A.V.; Larsen, L.B.; Hammershøj, M. Gel properties of potato protein and the isolated fractions of patatins and protease inhibitors—Impact of drying method, protein concentration, pH and ionic strength. Food Hydrocoll. 2019, 96, 246–258. [Google Scholar] [CrossRef]
- Dissanayake, M.; Ramchandran, L.; Piyadasa, C.; Vasiljevic, T. Influence of heat and pH on structure and conformation of whey proteins. Int. Dairy J. 2013, 28, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Dombrowski, J.; Gschwendtner, M.; Kulozik, U. Evaluation of structural characteristics determining surface and foaming properties of β-lactoglobulin aggregates. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 286–295. [Google Scholar] [CrossRef]
- Delahaije, R.J.B.M.; Wierenga, P.A.; van Nieuwenhuijzen, N.H.; Giuseppin, M.L.F.; Gruppen, H. Protein concentration and protein-exposed hydrophobicity as dominant parameters determining the flocculation of protein-stabilized oil-in-water emulsions. Langmuir 2013, 29, 11567–11574. [Google Scholar] [CrossRef] [PubMed]
- Vries, A.; Gomez, Y.L.; van der Linden, E.; Scholten, E. The effect of oil type on network formation by protein aggregates into oleogels. RSC Adv. 2017, 7, 11803–11812. [Google Scholar] [CrossRef] [Green Version]
- Wolz, M.; Kulozik, U. System parameters in a high moisture extrusion process for microparticulation of whey proteins. J. Food Eng. 2017, 209, 12–17. [Google Scholar] [CrossRef]
- Selmer, I.; Karnetzke, J.; Kleemann, C.; Lehtonen, M.; Mikkonen, K.S.; Kulozik, U.; Smirnova, I. Encapsulation of fish oil in protein aerogel micro-particles. J. Food Eng. 2019, 260, 1–11. [Google Scholar] [CrossRef]
- Betz, M.; Hörmansperger, J.; Fuchs, T.; Kulozik, U. Swelling behaviour, charge and mesh size of thermal protein hydrogels as influenced by pH during gelation. Soft Matter 2012, 8, 2477–2485. [Google Scholar] [CrossRef]
- Dachmann, E.; Nobis, V.; Kulozik, U.; Dombrowski, J. Surface and foaming properties of potato proteins: Impact of protein concentration, pH value and ionic strength. Food Hydrocoll. 2020, 107, 105981. [Google Scholar] [CrossRef]
- Katzav, H.; Chirug, L.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. Comparison of Thermal and High-Pressure Gelation of Potato Protein Isolates. Foods 2020, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Andlinger, D.J.; Bornkeßel, A.C.; Jung, I.; Schröter, B.; Smirnova, I.; Kulozik, U. Microstructures of potato protein hydrogels and aerogels produced by thermal crosslinking and supercritical drying. Food Hydrocoll. 2021, 112, 106305. [Google Scholar] [CrossRef]
- Racusen, D.; Weller, D.L. Molecular weight of Patatin, a major potato tuber protein. J. Food Biochem. 1984, 8, 103–107. [Google Scholar] [CrossRef]
- Rydel, T.J.; Williams, J.M.; Krieger, E.; Moshiri, F.; Stallings, W.C.; Brown, S.M.; Pershing, J.C.; Purcell, J.P.; Alibhai, M.F. The crystal structure, mutagenesis, and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry 2003, 42, 6696–6708. [Google Scholar] [CrossRef] [PubMed]
- Kurz, F.; Hengst, C.; Kulozik, U. RP-HPLC method for simultaneous quantification of free and total thiol groups in native and heat aggregated whey proteins. MethodsX 2020, 7, 101112. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M. β-Lactoglobulin heat denaturation: A critical assessment of kinetic modelling. Int. Dairy J. 2016, 52, 92–100. [Google Scholar] [CrossRef]
- Tolkach, A.; Kulozik, U. Reaction kinetic pathway of reversible and irreversible thermal denaturation of β-lactoglobulin. Lait 2007, 87, 301–315. [Google Scholar] [CrossRef] [Green Version]
- Anema, S.G.; Lee, S.K.; Klostermeyer, H. Effect of protein, nonprotein-soluble components, and lactose concentrations on the irreversible thermal denaturation of beta-lactoglobulin and alpha-lactalbumin in skim milk. J. Agric. Food. Chem. 2006, 54, 7339–7348. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Paterson, W.R.; Wilson, D.I. The pH threshold in the dissolution of beta-lactoglobulin gels and aggregates in alkali. Biomacromolecules 2007, 8, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Haug, I.J.; Skar, H.M.; Vegarud, G.E.; Langsrud, T.; Draget, K.I. Electrostatic effects on β-lactoglobulin transitions during heat denaturation as studied by differential scanning calorimetry. Food Hydrocoll. 2009, 23, 2287–2293. [Google Scholar] [CrossRef]
- Leeb, E.; Haller, N.; Kulozik, U. Effect of pH on the reaction mechanism of thermal denaturation and aggregation of bovine β-lactoglobulin. Int. Dairy J. 2018, 78, 103–111. [Google Scholar] [CrossRef]
- Handa, A.; Takashi, K.; Kuroda, N.; Froning, G.W. Heat-induced Egg White Gels as Affected by pH. J. Food Sci. 1998, 63, 403–407. [Google Scholar] [CrossRef]
- Baldwin, A.J. Insolubility of milk powder products—A minireview. Dairy Sci. Technol. 2010, 90, 169–179. [Google Scholar] [CrossRef]
- Martin, A.H.; Nieuwland, M.; Jong, G.A.H. Characterization of heat-set gels from RuBisCO in comparison to those from other proteins. J. Agric. Food. Chem. 2014, 62, 10783–10791. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Borderías, A.J.; Montero, P. Chemical Interactions of Nonmuscle Proteins in the Network of Sardine (Sardina pilchardus) Muscle Gels. LWT Food Sci. Technol. 1997, 30, 602–608. [Google Scholar] [CrossRef]
- Messens, W.; van de Walle, D.; Arevalo, J.; Dewettinck, K.; Huyghebaert, A. Rheological properties of high-pressure-treated Gouda cheese. Int. Dairy J. 2000, 10, 359–367. [Google Scholar] [CrossRef]
- Lupano, C.E. Gelation of mixed systems whey protein concentrate–gluten in acidic conditions. Food Res. Int. 2000, 33, 691–696. [Google Scholar] [CrossRef]
- Takenaka, O.; Aizawa, S.; Tamaura, Y.; Hirano, J.; Inada, Y. Sodium dodecyl sulfate and hydrophobic regions in bovine serum albumin. Biochim. Biophys. Acta Protein Struct. 1972, 263, 696–703. [Google Scholar] [CrossRef]
- Kato, A.; Matsuda, T.; Matsudomi, N.; Kobayashi, K. Determination of protein hydrophobicity using sodium dodecyl sulfate binding method. J. Agric. Food. Chem. 1984, 32, 284–288. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Tanford, C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc. Natl. Acad. Sci. USA 1970, 66, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Keim, S.; Hinrichs, J. Influence of stabilizing bonds on the texture properties of high-pressure-induced whey protein gels. Int. Dairy J. 2004, 14, 355–363. [Google Scholar] [CrossRef]
- Havea, P.; Watkinson, P.; Kuhn-Sherlock, B. Heat-induced whey protein gels: Protein-protein interactions and functional properties. J. Agric. Food. Chem. 2009, 57, 1506–1512. [Google Scholar] [CrossRef]
- Carrotta, R.; Bauer, R.; Waninge, R.; Rischel, C. Conformational characterization of oligomeric intermediates and aggregates in beta-lactoglobulin heat aggregation. Protein Sci. 2001, 10, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Carrotta, R.; Rischel, C.; Øgendal, L. Characterization and Isolation of Intermediates in β-Lactoglobulin Heat Aggregation at High pH. Biophys. J. 2000, 79, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Hua, Y.; Cui, S.W.; Wang, Q.; Mine, Y.; Poysa, V. Heat induced gelling properties of soy protein isolates prepared from different defatted soybean flours. Food Res. Int. 2005, 38, 377–385. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Dawson, K.A.; Wan, L. Thermal Aggregation of β-Lactoglobulin: Effect of pH, Ionic Environment, and Thiol Reagent1. J. Dairy Sci. 1993, 76, 70–77. [Google Scholar] [CrossRef]
- Boye, J.I.; Alli, I. Thermal denaturation of mixtures of α-lactalbumin and β-lactoglobulin: A differential scanning calorimetric study. Food Res. Int. 2000, 33, 673–682. [Google Scholar] [CrossRef]
- Broersen, K.; Van Teeffelen, A.M.M.; Vries, A.; Voragen, A.G.J.; Hamer, R.J.; De Jongh, H.H.J. Do Sulfhydryl Groups Affect Aggregation and Gelation Properties of Ovalbumin? J. Agric. Food. Chem. 2006, 54, 5166–5174. [Google Scholar] [CrossRef] [PubMed]
- Cairoli, S.; Iametti, S.; Bonomi, F. Reversible and irreversible modifications of β-lactoglobulin upon exposure to heat. J. Protein Chem. 1994, 13, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Delahaije, R.J.B.M.; Wierenga, P.A.; Giuseppin, M.L.F.; Gruppen, H. Improved emulsion stability by succinylation of patatin is caused by partial unfolding rather than charge effects. J. Colloid Interface Sci. 2014, 430, 69–77. [Google Scholar] [CrossRef]
- Lan, H.; Liu, H.; Ye, Y.; Yin, Z. The Role of Surface Properties on Protein Aggregation Behavior in Aqueous Solution of Different pH Values. AAPS PharmSciTech 2020, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.H.; Wright, P.E.; Scheraga, H.A. The role of hydrophobic interactions in initiation and propagation of protein folding. Proc. Natl. Acad. Sci. USA 2006, 103, 13057–13061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Seeman, D.; Zheng, B.; Kizilay, E.; Xu, Y.; Dubin, P.L. pH-Dependent aggregation and disaggregation of native β-lactoglobulin in low salt. Langmuir 2013, 29, 4584–4593. [Google Scholar] [CrossRef]









| pH Value | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|
| peak maximum (Td) | 64.5 ± 0.2 | 60.8 ± 0.4 | 57.7 ± 0.6 | 54.8 ± 0.1 | 51.5 ± 0.6 |
| pH 6 | pH 7 | pH 8 | pH 9 | pH 10 | |
|---|---|---|---|---|---|
| Td − 10 °C | 6.09 ± 0.30 | 8.33 ± 0.39 | 9.57 ± 0.58 | 7.75 ± 0.98 | 8.27 ± 0.57 |
| Td − 5 °C | 3.45 ± 0.10 | 4.96 ± 0.11 | 5.37 ± 0.13 | 5.55 ± 0.16 | 6.49 ± 0.28 |
| Td + 5 °C | 1.10 ± 0.24 | 1.89 ± 0.32 | 2.19 ± 0.30 | 2.42 ± 0.31 | 2.32 ± 0.31 |
| Td + 10 °C | 1.48 ± 0.27 | 1.69 ± 0.36 | 2.29 ± 0.40 | 3.02 ± 0.46 | 2.60 ± 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andlinger, D.J.; Röscheisen, P.; Hengst, C.; Kulozik, U. Influence of pH, Temperature and Protease Inhibitors on Kinetics and Mechanism of Thermally Induced Aggregation of Potato Proteins. Foods 2021, 10, 796. https://doi.org/10.3390/foods10040796
Andlinger DJ, Röscheisen P, Hengst C, Kulozik U. Influence of pH, Temperature and Protease Inhibitors on Kinetics and Mechanism of Thermally Induced Aggregation of Potato Proteins. Foods. 2021; 10(4):796. https://doi.org/10.3390/foods10040796
Chicago/Turabian StyleAndlinger, David J., Pauline Röscheisen, Claudia Hengst, and Ulrich Kulozik. 2021. "Influence of pH, Temperature and Protease Inhibitors on Kinetics and Mechanism of Thermally Induced Aggregation of Potato Proteins" Foods 10, no. 4: 796. https://doi.org/10.3390/foods10040796