Microbial Behavior and Influencing Factors in the Anaerobic Digestion of Distiller: A Comprehensive Review
Abstract
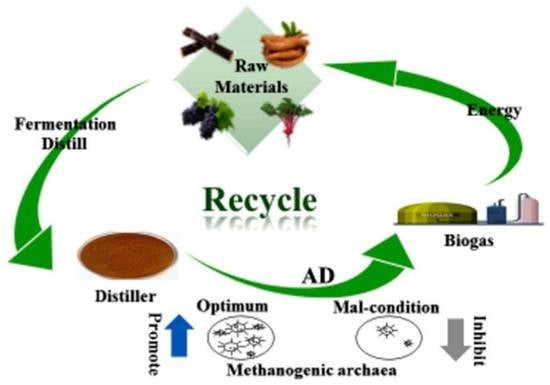
:1. Introduction
2. Present Situation of Distiller
2.1. Distiller Production and Characterization
2.2. Different Treatment Methods of Distiller
2.3. Potential Energy and the Synergy of Co-Digestion
3. Analysis Based on Microbial Perspective
3.1. Methanogenesis Pathway and Parameter Influence
3.1.1. Hydrogenotrophic Methanogenesis
3.1.2. Acetotrophic Pathway
3.2. Stability of System
3.3. Methanogenic Archaea
3.3.1. Diversity of Methanogenic Archaea
3.3.2. Effects of Operating Parameters on Archaeal Community
pH
Ammonia/Ammonium
Organic Load Rate and Hydraulic Retention Time
Hydrogen
VFAs
Temperature
Sulfur
Metal Ions
4. Archaea and Process Performance
4.1. Methanobacterium
4.2. Methanosaeta
4.3. Methanosarcina
4.4. Methanoculleus
5. Removal of Harmful Ingredients
5.1. Melanoidins
5.2. Phenolic Compounds
5.3. Sulfur
5.3.1. Removal of H2S from Biogas
5.3.2. Removal of Sulfate and Sulfide
5.3.3. Inhibition of Sulfate-Reducing Bacteria Activity
6. Future Prospects for Distiller Treatment
6.1. Integration with Membrane Separation Technology
6.2. Inactivated Pathogens
6.3. Coupling with Built-In Microbial Batteries
6.4. Pretreatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Substrate | Optimal Proportion | Synergy | Energy Potential of Distillers | Ref. |
|---|---|---|---|---|
| sewage sludge wine vinasse poultry manure | 49.5:49.5:1 | 1. pH is stable at neutral 2. The process effect is best at short HRT (13 days) | With the addition of wine vinasse, the process performance improved and methane production increased | [13] |
| 1. sugar beet vinasse 2. sugar beet vinasse, cow manure 3. sugar beet vinasse, straw. | 1. AD of sugar beet vinasse alone was not possible 2. The average values were 323.1 ± 48.6 mL CH4 g VS−1 3. The average values were 287.7 ± 22.2 mL CH4 g VS−1 | 1. BMP of sugar beet vinasse is 267.4 ± 4.5 L CH4 kg VS−1 2. Average VS/DM ratio is 71.5 ± 2.5% | [33] | |
| sugar beet pulp silage molasses vinasse | 3:1 | 1. The highest biogas productivity (598.1 mL/g VS) was achieved at the SBPS–vinasse ratio of 3:1 2. Biogas yield of SBPS and vinasse fermented alone decreased by 13% and 28.6%, respectively | SBPS contained 18.71% TS and 93.8% VS vinasse (6.75% TS and 75.11% VS) | [37] |
| sugarcane vinasse filter cake deacetylation liquor | Organic matter removal rate and methane production were the highest | High volatile fatty acid content | [38] | |
| sewage sludge wine vinasse poultry manure | 49.5:49.5:1 | 1. The co-digestion of three substrates was more advantageous 2. Relieves the inhibition of the high TAN | BMP increases with the addition of wine vinasse | [47] |
| sludge wine vinasse poultry manure | 49.5:49.5:1. | 1. In the experiment of 10 g/L poultry manure, H2 production, and biogas production were 18.20% and 27.57% higher, respectively, compared with 20 g/L. 2. The accumulation of TAN and FAN was alleviated | BHP tests: 22.34 mL H2/g SCOD and 27.1 mL H2/g VS | [48] |
| sludge wine vinasse | 25:75 | Under the optimum ratio, the highest hydrogen yield was 43.25 ± 1.52 mL H2/g VS, which was 14 times higher than that of sludge single fermentation | With the increase of wine vinasse in the mixture, hydrogen production increased significantly | [49] |
| sugarcane vinasse filter cake deacetylation liquor | 1. Compared with traditional AD, energy efficiency is improved by at least 16% 2. pH is stable at neutral | Single vinasse has high BMP, the mixture of BMP increased by about 38% | [50] | |
| sludge wine vinasse poultry manure | 49.5:49.5:1 | The VS and total volatile fatty acids reached, respectively, 93.13% and 97.43% of removal efficiency | With the addition of wine vinasse, the process performance improved and methane production increased | [51] |
| FOG slaughterhouse wastewater | FOG concentrations are 5–10% | 1. Methane production increased by a factor of two to five 2. The biodegradability of almost all substrates was improved | [52] | |
| food waste chicken manure | 1. Co-digestion improves system productivity for almost all parameters 2. The synergistic effect of microorganisms was obvious | [53] | ||
| sugarcane vinasse urea trace elements | 1. Stable operation under high OLR and low HRT 2. 79% higher methane production rate with a stable specific methane production of 239 mL g COD−1 | [54] | ||
| cow dung anaerobic granular sludge activated sludge food waste | 1:1:1:1 | 1. Optimal co-digested inoculum for biological methanation 2. The kinetics of organic degradation produces a scaling effect | [55] | |
| sugarcane vinasse chicken manure | 3:1 | 1. Co-digestion relieved the inhibition of TVFA and TAN 2. Co-digestion had the highest hydrolytic activity | BMP increased with the increase of vinasse | [56] |
| sewage sludge Sherry-wine distillery wastewater | 1:1 | 1. High methane production:154 L CH4/kg COD 2. The archaea were enriched in co-digestion | [57] |
| Order | Type | Available Substrate | Other Features | |
|---|---|---|---|---|
| Methanomicrobiales | hydrogenotrophic methanogens | H2 | Little affected by temperature and acetic acid concentration It is negatively correlated with SO42−/COD Concentrations of high total ammonia and salt showed a clearer effect than Methanobacteriales Higher acid resistance than Methanosaeta sp., Methanobacteriales, Methanococcales | [25] [27] [69] [104] |
| Methanobacteriales | hydrogenotrophic methanogens | H2, CO2, formic acid | Strictly hydrogenotrophic methanogens’; syntrophic acetate oxidation and hydrogenotrophic methanogenesis Greatly affected by temperature and acetic acid concentration When SO42−/COD is 0.05, the dominant bacterial order; it is greatly influenced by SO42−/COD and negatively correlated It is widely distributed and positively correlated with VFA, OLR, and temperature Optimal methanogenesis at optimal OLR (17.05 kg COD/m3-day) | [6] [25] [27] [69] [93] |
| Methanococcales | H2 | Cover most methanogens encountered in anaerobic digesters | [104] | |
| Methanosarcinales | Greatly affected by acetic acid concentration and not sensitive to temperature Optimal methanogenesis at optimal OLR (17.05 kg COD/m3-day) | [25] [93] | ||
| Family | ||||
| Methanosaetaceae | acetate | High acetic acid concentration is suitable for growth and inversely proportional to temperature In contrast to Methanomicrobiales and Methanobacteriales, the ratio of SO42−/COD has little influence Methanosaetaceae was negatively correlated with FAN, total ammonia, volatile fatty acids, and conductivity; however, it was positively related to Methanosarcinaceae and played a crucial role in low total ammonia, salt, and volatile fatty acid In general, a filamentous shape | [25] [27] [29] [69]. | |
| Methanosarcinaceae | acetic acid, methanol, other methylated C1 compounds, H2, CO2, CO | When growing on acetic acid, it is greatly affected by temperature Methanosarcinaceae was positively correlated with Methanosaetaceae and negatively correlated with total ammonia, volatile fatty acids, and conductivity; Methanosarcinaceae have a spherical form Multicellular clusters Relative abundance increased with increasing TAN Plays a key role in promoting methane production from biological carbon The most robust methanogen from metabolic and physiologic points May grow on the C1 compound in the absence of hydrogen | [25] [29] [69] [68] [75] [119] [120] | |
| Genus | ||||
| Methanobacterium | hydrogenotrophic methanogens | CO2, H2, formic acid | The relative abundance increases with the increase of OLR Frequent interaction with the outside world and high acid and ammonia resistance Mainly in mesophilic temperatures(30–35 °C) Methanobacterium had no obvious influence on mono-digestion and co-digestion. Relative abundance increased with increasing hydrogen concentration Little affected by potassium concentration Relative abundance was positively correlated with low ammonia concentration and negatively correlated with high ammonia concentration Little change in long-term competition with SRB It decreases with the addition of CTS | [16] [29] [38] [39] [67] [72] [68] [112] [134] |
| Methanosaeta | acetoclastic methanogens | acetate | Decreased relative abundance under high OLR and high acetic acid In 20 g-COD/L acetate reactors, the relative abundance at high temperatures decreased, accounting for only 0.07% of the archaea community FAN, pH, and TA were all negatively correlated with Methanosaeta Methanosaeta was more sensitive to high concentrations of acetate than Methanosarcina Halt-tolerant Archaea Under the condition of rich hydrogen, decrease; Active acetyl nutrient methanogenesis at high ammonia concentrations Lower affinity for acetate than Methanosarcina When the TAN level was lower than 560 mg/L, Methanosaeta was more active and not inhibited by ammonia; the activity is inversely proportional to the concentration of ammonium and acetic acid At high COD/SO42−ratios, playing a major role With the decrease of COD/SO42−, the quantity increases and the growth rate is higher than Methanosarcina The relative abundance of Methanosaeta increases dramatically during long-term competition with SRB At high OLR, the activity is severely inhibited, which can be alleviated by adding CAW; A broken shell methanogen; Slow-growing; Addition of CTS increases relative abundance | [16] [25] [29] [39] [58] [67] [72] [68] [93,97] [95] [112] [134] |
| Methanosarcina | mixotrophic methanogen | methanol, methylamine acetate H2, CO2 | Decreased relative abundance at high OLR and high acetic acid Feeding acetate at a concentration of 10 mM resulted in enrichment At lower acetate concentrations (20 g-COD/L), dominant methanogen, 84%(mesophilic) and 88% (thermophilic) At high ammonia concentrations, acetate cannot be efficiently metabolized It is negatively correlated with ammonia concentration, but the tolerance concentration of ammonia is as high as 7000 mg/L; survives in a weakly acidic environment Methanosarcina disappears at phenol concentration of 5 g/L Grows slowly on acetate With the decrease of COD/SO42−, the quantity increases, and the growth rate is lower than Methanosaeta Involved in multiple methanogenic pathways Drop after adding CTS It was involved in the recovery after inhibitory events with high levels of acetate The effect of Methanosarcina was more obvious under thermo-alkali pretreatment | [16] [24] [25] [29] [68] [79] [92] [95] [103] [134] [113] [115] |
| Methanoculleus | hydrogenotrophic methanogens | mainly H2, CO2 | At medium temperature, plays an important role; The relative abundance increased with the concentration of acetate, and the increase in temperature Major genus of thermophilic processes, capable of co-trophic acetic acid oxidation (SAO) and hydrotrophic methanogenesis Methanoculleus is a mesophilicarchaea; may survive in a weakly acidic environment Disappear when phenol concentration is 5 g/L With the increase in phenol concentration (0.50 g/L to 2.00 g/L), it became the dominant community | [25] [38] [68] [79] [113] |
| Methanothermobacter | hydrogenotrophic methanogens | H2, CO2 | In high acetic acid concentrations (60 g-COD/L) and high temperatures become the dominant community (94% of the archaeal community); Participate in the SAO-HM pathway Major Genera in thermophilic processes Emerged in the long-running competition with SRB | [25] [38] [119] |
| Methanoregula | hydrogenotrophic methanogens | H2 | Disappeared from the long competition with SRB | [119] |
| Methanofollis | H2 | Disappeared from the long competition with SRB | [119] | |
| Methanospirillum | hydrogenotrophic methanogens | Disappear as the temperature rises When the concentration of ammonium accumulates to 6 g/L, the metabolism is inhibited Addition of CTS increases relative abundance | [25] [68] [134] | |
| Methanobrevibacter | hydrogenotrophic methanogens | H2, CO2, formate | Increases with the increase of hydrogen Relative abundance decreased with addition of CTS | [36] [134] |
| Species | ||||
| Methanosaeta concilii | acetoclastic methanogen | acetate | The optimal sodium concentration is <60 mM It does not change with COD/SO42 and plays an important role in the consumption of acetate as an energy source for methane production Reduced in long-term competition with SRB (40.0%-15.2%) | [72] [97] [112] |
| Methanosaeta thermophile | acetoclastic methanogen | acetate | The optimal sodium concentration is <130 mM Disappeared with the decrease of COD/SO42− | [72] [97] |
| Methanosaeta harundinacea | acetoclastic methanogen | acetate | The optimal sodium concentration is <20 mM It increases slightly with the decrease of COD/SO42− Significant increase in long-term competition with SRB (2.5–35.2%) | [72] [97] [112] |
| Methanoregula formicicum | hydrogenotrophic methanogen | H2 | Disappeared from the long competition with SRB | [112] |
| Methanoregula boonei | hydrogenotrophic methanogen | H2 | Disappeared from the long competition with SRB | [112] |
| Methanobacterium petrolearium | H2 | Emerging in long-term competition with SRB (0–6.9%) | [112] | |
| Methanobacterium beijingense | H2 | Salt tolerance Emerging in long-term competition with SRB (0–4.8%) | [58] [112] | |
| Methanothermobacter tenebrarum | H2 | Emerging from a long rivalry with the SRB | [112] | |
| Methanosarcina mazei | acetate | Occurs in long-term competition with SRB, but in low relative abundance | [112] | |
| Methanosarcina acetivorans | M. acetivorans may grow nonmethanogenically, using CO as a substrate | [103] | ||
| Methanomethylovoran hollandica | methanol, methylamines acetate | Occurs in long-term competition with SRB, but in low relative abundance | [112] | |
| Methanofollis liminatans | hydrogenotrophic methanogen | H2 | Occurs in long-term competition with SRB, but in low relative abundance | [112] |
References
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing bioenergy production from the raw and defatted microalgal biomass using wastewater as the cultivation medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hossen, E.H.; Aziz, T.N.; Ducoste, J.J.; de Los Reyes, F.L. Increased loading stress leads to convergence of microbial communities and high methane yields in adapted anaerobic co-digesters. Water Res. 2020, 169, 115155. [Google Scholar] [CrossRef] [PubMed]
- Toshinari, M.; Sarah, S.; Viviana, S.; Yuki, H.; Shotaro, T. Engineering anaerobic digestion via optimizing microbial community: Efects of bactericidal agents, quorum sensing inhibitors, and inorganic materials. Appl. Microbiol. Biotechnol. 2021, 105, 7607–7618. [Google Scholar]
- Janke, L.; Leite, A.; Nikolausz, M.; Schmidt, T.; Liebetrau, J.; Nelles, M.; Stinner, W. Biogas Production from Sugarcane Waste: Assessment on Kinetic Challenges for Process Designing. Int. J. Mol. Sci. 2015, 16, 20685–20703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, N.; Milandile, M.H.; Quan, C.; Rundong, L. Critical assessment of plasma tar reforming during biomass gasification: A review on advancement in plasma technology. J. Hazard. Mater. 2022, 421, 126764. [Google Scholar] [CrossRef] [PubMed]
- Rachbauer, L.; Beyer, R.; Bochmann, G.; Fuchs, W. Characteristics of adapted hydrogenotrophic community during biomethanation. Sci. Total Environ. 2017, 595, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Jin, K.; Yi, R.; Chen, M.; Peng, J.; Pan, Y. Enhancement of bioenergy recovery from agricultural wastes through recycling of cellulosic alcoholic fermentation vinasse for anaerobic co-digestion. Bioresour. Technol. 2020, 311, 123511. [Google Scholar] [CrossRef]
- Hoarau, J.; Caro, Y.; Grondin, I.; Petit, T. Sugarcane vinasse processing: Toward a status shift from waste to valuable resource. A review. J. Water Process Eng. 2018, 24, 11–25. [Google Scholar] [CrossRef]
- Buller, L.S.; Romero, C.; Lamparelli, R.A.C.; Ferreira, S.F.; Bortoleto, A.P.; Mussatto, S.I.; Forster-Carneiro, T. A spatially explicit assessment of sugarcane vinasse as a sustainable by-product. Sci. Total Environ. 2021, 765, 142717. [Google Scholar] [CrossRef]
- Lopes, S.I.; Capela, M.I.; Lens, P.N. Sulfate reduction during the acidification of sucrose at pH 5 under thermophilic (55 degrees C) conditions. II: Effect of sulfide and COD/ SO42-ratio. Bioresour. Technol. 2010, 101, 4278–4284. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Brasil, Y.L.; Koch, K.; Amaral, M.C.S. Resource recovery from sugarcane vinasse by anaerobic digestion—A review. J. Environ. Manag. 2021, 295, 113137. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.C.; Leigh, J.A. Metabolic versatility in methanogens. Curr. Opin. Biotechnol. 2014, 29, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sillero, L.; Solera, R.; Perez, M. Improvement of the anaerobic digestion of sewage sludge by co-digestion with wine vinasse and poultry manure: Effect of different hydraulic retention times. Fuel 2022, 321, 124104. [Google Scholar] [CrossRef]
- Rodrigues Reis, C.E.; Hu, B. Vinasse from Sugarcane Ethanol Production: Better Treatment or Better Utilization? Front. Energy Res. 2017, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.F.R.; Magalhaes, N.C.; Cunha, P.V.M.; Amaral, M.C.S.; Koch, K. Influence of COD/SO42-ratio on vinasse treatment performance by two-stage anaerobic membrane bioreactor. J. Environ. Manag. 2020, 259, 110034. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Cervantes, A.; Guevara-Santos, N.; Arreola-Vargas, J.; Snell-Castro, R.; Méndez-Acosta, H.O. Performance and microbial dynamics in packed-bed reactors during the long-term two-stage anaerobic treatment of tequila vinasses. Biochem. Eng. J. 2018, 138, 12–20. [Google Scholar] [CrossRef]
- Chowdhary, P.; Raj, A.; Bharagava, R.N. Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environmental threats: A review. Chemosphere 2018, 194, 229–246. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem. 2019, 84, 81–90. [Google Scholar] [CrossRef]
- Bork, L.V.; Haase, P.T.; Rohn, S.; Kanzler, C. Structural characterization of polar melanoidins deriving from Maillard reaction intermediates—A model approach. Food Chem. 2022, 395, 133592. [Google Scholar] [CrossRef]
- da Silva Neto, J.V.; Gallo, W.L.R.; Nour, E.A.A. Production and use of biogas from vinasse: Implications for the energy balance and GHG emissions of sugar cane ethanol in the brazilian context. Environ. Prog. Sustain. Energy 2019, 39, 13226. [Google Scholar] [CrossRef]
- Peiter, F.S.; Hankins, N.P.; Pires, E.C. Evaluation of concentration technologies in the design of biorefineries for the recovery of resources from vinasse. Water Res. 2019, 157, 483–497. [Google Scholar] [CrossRef]
- Fuess, L.T.; Garcia, M.L. Bioenergy from stillage anaerobic digestion to enhance the energy balance ratio of ethanol production. J. Environ. Manag. 2015, 162, 102–114. [Google Scholar] [CrossRef]
- Fuess, L.T.; Garcia, M.L. Implications of stillage land disposal: A critical review on the impacts of fertigation. J. Environ. Manag. 2014, 145, 210–229. [Google Scholar] [CrossRef]
- Dyksma, S.; Jansen, L.; Gallert, C. Syntrophic acetate oxidation replaces acetoclastic methanogenesis during thermophilic digestion of biowaste. Microbiome 2020, 8, 105. [Google Scholar] [CrossRef]
- Zhao, J.; Westerholm, M.; Qiao, W.; Yin, D.; Bi, S.; Jiang, M.; Dong, R. Impact of temperature and substrate concentration on degradation rates of acetate, propionate and hydrogen and their links to microbial community structure. Bioresour. Technol. 2018, 256, 44–52. [Google Scholar] [CrossRef]
- Ramos, L.R.; Lovato, G.; Rodrigues, J.A.D.; Silva, E.L. Anaerobic digestion of vinasse in fluidized bed reactors: Process robustness between two-stage thermophilic-thermophilic and thermophilic-mesophilic systems. J. Clean. Prod. 2021, 314, 128066. [Google Scholar] [CrossRef]
- Jiménez, J.; Barrera, E.L.; De Vrieze, J.; Boon, N.; DeMeester, S.; Spanjers, H.; Romero, O.R.; Dewulf, J. Microbial community dynamics reflect reactor stability during the anaerobic digestion of a very high strength and sulfate-rich vinasse. J. Chem. Technol. Biotechnol. 2018, 93, 975–984. [Google Scholar] [CrossRef]
- Cremonez, P.A.; Teleken, J.G.; Weiser Meier, T.R.; Alves, H.J. Two-Stage anaerobic digestion in agroindustrial waste treatment: A review. J. Environ. Manag. 2021, 281, 111854. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, S.; Li, L.; Zhao, X.; Ma, Y.; Shi, D. Long-term high-solids anaerobic digestion of food waste: Effects of ammonia on process performance and microbial community. Bioresour. Technol. 2018, 262, 148–158. [Google Scholar] [CrossRef]
- Prangya, R.; Daya, S.; Macsen, H.; Caitlin, B.; Helmer, L.; Reddicherla, U.; Sanjay, M.; Sagarika, P.; Mukesh, G. Sustainable Valorisation of Animal Manures via Thermochemical Conversion Technologies: An Inclusive Review on Recent Trends. Waste Biomass Valorization 2022, 14, 553–582. [Google Scholar]
- Umapathi, R.; Park, B.; Sonwal, S.; Rani, G.M.; Cho, Y.J.; Huh, Y. Advances in optical-sensing strategies for the on-site detection of pesticides in agricultural foods. Trends Food Sci. Technol. 2022, 119, 69–89. [Google Scholar] [CrossRef]
- Santana Junior, A.E.; Duda, R.M.; Oliveira, R.A.D. Improving the energy balance of ethanol industry with methane production from vinasse and molasses in two-stage anaerobic reactors. J. Clean. Prod. 2019, 238, 117577. [Google Scholar] [CrossRef]
- Moraes, B.S.; Triolo, J.M.; Lecona, V.P.; Zaiat, M.; Sommer, S.G. Biogas production within the bioethanol production chain: Use of co-substrates for anaerobic digestion of sugar beet vinasse. Bioresour. Technol. 2015, 190, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane vinasse as organo-mineral fertilizers feedstock: Opportunities and environmental risks. Sci. Total Environ. 2022, 832, 154998. [Google Scholar] [CrossRef]
- Sydney, E.B.; Carvalho, J.C.; Letti, L.A.J.; Magalhaes, A.I., Jr.; Karp, S.G.; Martinez-Burgos, W.J.; Candeo, E.S.; Rodrigues, C.; Vandenberghe, L.P.S.; Neto, C.J.D.; et al. Current developments and challenges of green technologies for the valorization of liquid, solid, and gaseous wastes from sugarcane ethanol production. J. Hazard. Mater 2021, 404, 124059. [Google Scholar] [CrossRef]
- Buenavista, R.M.E.; Siliveru, K.; Zheng, Y. Utilization of Distiller’s dried grains with solubles: A review. J. Agric. Food Res. 2021, 5, 100195. [Google Scholar] [CrossRef]
- Zieminski, K.; Kowalska-Wentel, M. Effect of enzymatic pretreatment on anaerobic co-digestion of sugar beet pulp silage and vinasse. Bioresour. Technol. 2015, 180, 274–280. [Google Scholar] [CrossRef]
- Volpi, M.P.C.; Junior, A.; Franco, T.T.; Moraes, B.S. Operational and biochemical aspects of co-digestion (co-AD) from sugarcane vinasse, filter cake, and deacetylation liquor. Appl. Microbiol. Biotechnol. 2021, 105, 8969–8987. [Google Scholar] [CrossRef]
- Jiang, Q.; Xin, Y.; Jiang, Y.; Huang, L.; Shen, P. Improving the efficiency of anaerobic digestion of Molasses alcohol wastewater using Cassava alcohol wastewater as a mixed feedstock. Bioresour. Technol. 2022, 344, 126179. [Google Scholar] [CrossRef]
- Mendez-Acosta, H.O.; Snell-Castro, R.; Alcaraz-Gonzalez, V.; Gonzalez-Alvarez, V.; Pelayo-Ortiz, C. Anaerobic treatment of Tequila vinasses in a CSTR-type digester. Biodegradation 2010, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Oñate, J.; Arenas, A.; Ruiz, A.; Rivera, K.; Peláez, C. Evaluation of Mutagenic and Genotoxic Activity in Vinasses Subjected to Different Treatments. Water Air Soil. Pollut. 2015, 226, 144. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wang, H.; Zhao, X.; Cui, H.; Wei, Z. Reducing nitrogen loss and phytotoxicity during beer vinasse composting with biochar addition. Waste Manag. 2017, 61, 150–156. [Google Scholar] [CrossRef]
- Fuess, L.T.; Garcia, M.L.; Zaiat, M. Seasonal characterization of sugarcane vinasse: Assessing environmental impacts from fertirrigation and the bioenergy recovery potential through biodigestion. Sci. Total Environ. 2018, 634, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Tena, M.; Buller, L.S.; Sganzerla, W.G.; Berni, M.; Forster-Carneiro, T.; Solera, R.; Pérez, M. Techno-economic evaluation of bioenergy production from anaerobic digestion of by-products from ethanol flex plants. Fuel 2022, 309, 122171. [Google Scholar] [CrossRef]
- Longati, A.A.; Lino, A.R.A.; Giordano, R.C.; Furlan, F.F.; Cruz, A.J.G. Biogas Production from Anaerobic Digestion of Vinasse in Sugarcane Biorefinery: A Techno-economic and Environmental Analysis. Waste Biomass Valorization 2019, 11, 4573–4591. [Google Scholar] [CrossRef]
- Kondusamy, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high rate methane production—A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Biochemical assays of potential methane to test biogas production from dark fermentation of sewage sludge and agricultural residues. Int. J. Hydrogen Energy 2022, 47, 13289–13299. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Perez, M. Anaerobic co-digestion of sewage sludge, wine vinasse and poultry manure for bio-hydrogen production. Int. J. Hydrogen Energy 2022, 47, 3667–3678. [Google Scholar] [CrossRef]
- Tena, M.; Luque, B.; Perez, M.; Solera, R. Enhanced hydrogen production from sewage sludge by cofermentation with wine vinasse. Int. J. Hydrogen Energy 2020, 45, 15977–15984. [Google Scholar] [CrossRef]
- Volpi, M.P.C.; Brenelli, L.B.; Mockaitis, G.; Rabelo, S.C.; Franco, T.T.; Moraes, B.S. Use of Lignocellulosic Residue from Second-Generation Ethanol Production to Enhance Methane Production Through Co-digestion. BioEnergy Res. 2021, 15, 602–616. [Google Scholar] [CrossRef]
- Sillero, L.; Solera, R.; Pérez, M. Thermophilic-mesophilic temperature phase anaerobic co-digestion of sewage sludge, wine vinasse and poultry manure: Effect of hydraulic retention time on mesophilic-methanogenic stage. Chem. Eng. J. 2023, 451, 138478. [Google Scholar] [CrossRef]
- Agabo-Garcia, C.; Solera, R.; Perez, M. First approaches to valorizate fat, oil and grease (FOG) as anaerobic co-substrate with slaughterhouse wastewater: Biomethane potential, settling capacity and microbial dynamics. Chemosphere 2020, 259, 127474. [Google Scholar] [CrossRef] [PubMed]
- Chuenchart, W.; Logan, M.; Leelayouthayotin, C.; Visvanathan, C. Enhancement of food waste thermophilic anaerobic digestion through synergistic effect with chicken manure. Biomass Bioenergy 2020, 136, 105541. [Google Scholar] [CrossRef]
- Janke, L.; Leite, A.F.; Batista, K.; Silva, W.; Nikolausz, M.; Nelles, M.; Stinner, W. Enhancing biogas production from vinasse in sugarcane biorefineries: Effects of urea and trace elements supplementation on process performance and stability. Bioresour. Technol. 2016, 217, 10–20. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Suthar, S. Anaerobic digestion of activated sludge, anaerobic granular sludge and cow dung with food waste for enhanced methane production. J. Clean. Prod. 2017, 164, 557–566. [Google Scholar] [CrossRef]
- Marin Batista, J.D.; Salazar, L.; Castro, L.; Escalante, H. Co-digestión anaerobia de vinaza y gallinaza de jaula: Alternativa para el manejo de residuos agrícolas colombianos. Rev. Colomb. Biotecnol. 2016, 18, 6–12. [Google Scholar] [CrossRef]
- Ripoll, V.; Agabo-García, C.; Perez, M.; Solera, R. Improvement of biomethane potential of sewage sludge anaerobic co-digestion by addition of “sherry-wine” distillery wastewater. J. Clean. Prod. 2020, 251, 119667. [Google Scholar] [CrossRef]
- Lefebvre, O.; Quentin, S.; Torrijos, M.; Godon, J.J.; Delgenes, J.P.; Moletta, R. Impact of increasing NaCl concentrations on the performance and community composition of two anaerobic reactors. Appl. Microbiol. Biotechnol. 2007, 75, 61–69. [Google Scholar] [CrossRef]
- Cabrera-Diaz, A.; Pereda-Reyes, I.; Oliva-Merencio, D.; Lebrero, R.; Zaiat, M. Anaerobic Digestion of Sugarcane Vinasse Through a Methanogenic UASB Reactor Followed by a Packed Bed Reactor. Appl. Biochem. Biotechnol. 2017, 183, 1127–1145. [Google Scholar] [CrossRef]
- De Vrieze, J.; De Lathouwer, L.; Verstraete, W.; Boon, N. High-rate iron-rich activated sludge as stabilizing agent for the anaerobic digestion of kitchen waste. Water Res. 2013, 47, 3732–3741. [Google Scholar] [CrossRef]
- Wang, B.; Stromberg, S.; Li, C.; Nges, I.A.; Nistor, M.; Deng, L.; Liu, J. Effects of substrate concentration on methane potential and degradation kinetics in batch anaerobic digestion. Bioresour. Technol. 2015, 194, 240–246. [Google Scholar] [CrossRef]
- Zhang, W.; Werner, J.J.; Agler, M.T.; Angenent, L.T. Substrate type drives variation in reactor microbiomes of anaerobic digesters. Bioresour. Technol. 2014, 151, 397–401. [Google Scholar] [CrossRef]
- Kiani Deh Kiani, M.; Parsaee, M.; Safieddin Ardebili, S.M.; Reyes, I.P.; Fuess, L.T.; Karimi, K. Different bioreactor configurations for biogas production from sugarcane vinasse: A comprehensive review. Biomass Bioenergy 2022, 161, 106446. [Google Scholar] [CrossRef]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic membrane bioreactors for wastewater treatment: Novel configurations, fouling control and energy considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Harihastuti, N.; Yuliasni, R.; Djayanti, S.; Handayani, N.; Rame, R.; Prasetio, A.; Kadier, A. Full-Scale Application of Up-flow High Rate Anaerobic Reactor with Substrate Modification and Effluent Recirculation for Sugarcane Vinasse Degradation and Biogas Generation. J. Ecol. Eng. 2021, 22, 314–324. [Google Scholar] [CrossRef]
- Mostafa, P.; Mostafa, K.D.; Keikhosro, K. A review of biogas production from sugarcane vinasse. Biomass Bioenerg. 2019, 122, 117–125. [Google Scholar]
- Wu, B.; He, C.; Yuan, S.; Hu, Z.; Wang, W. Hydrogen enrichment as a bioaugmentation tool to alleviate ammonia inhibition on anaerobic digestion of phenol-containing wastewater. Bioresour. Technol. 2019, 276, 97–102. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Xue, L.; Chen, C.; Liu, G.; Zhang, R. Effects of Ammonia on Anaerobic Digestion of Food Waste: Process Performance and Microbial Community. Energy Fuels 2016, 30, 5749–5757. [Google Scholar] [CrossRef]
- De Vrieze, J.; Saunders, A.M.; He, Y.; Fang, J.; Nielsen, P.H.; Verstraete, W.; Boon, N. Ammonia and temperature determine potential clustering in the anaerobic digestion microbiome. Water Res. 2015, 75, 312–323. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Ling, T.C.; Juan, J.C.; Lee, D.J.; Chang, J.S.; Show, P.L. Biorefineries of carbon dioxide: From carbon capture and storage (CCS) to bioenergies production. Bioresour. Technol. 2016, 215, 346–356. [Google Scholar] [CrossRef]
- Karadagli, F.; Rittmann, B.E. Kinetic characterization of Methanobacterium bryantii MoH. Environ. Sci. Technol. 2005, 39, 4900–4905. [Google Scholar] [CrossRef]
- Onodera, T.; Syutsubo, K.; Hatamoto, M.; Nakahara, N.; Yamaguchi, T. Evaluation of cation inhibition and adaptation based on microbial activity and community structure in anaerobic wastewater treatment under elevated saline concentration. Chem. Eng. J. 2017, 325, 442–448. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, M.; Chen, Y.; Yu, M.; Ruan, W. Tolerance response to in situ ammonia stress in a pilot-scale anaerobic digestion reactor for alleviating ammonia inhibition. Bioresour. Technol. 2015, 198, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Escalante, F.M.; Pelayo-Ortíz, C.; Navarro-Corona, J.; González-García, Y.; Bories, A.; Gutiérrez-Pulido, H. Anaerobic digestion of the vinasses from the fermentation of Agave tequilana Weber to tequila: The effect of pH, temperature and hydraulic retention time on the production of hydrogen and methane. Biomass Bioenergy 2009, 33, 14–20. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, F.; Xu, H.; Feng, D.; Liu, J.; Han, G. Biochar promotes methane production at high acetate concentrations in anaerobic soils. Environ. Chem. Lett. 2019, 17, 1347–1352. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Abbas, B.; Geleijnse, M.; Kolganova, T.V.; Kleerebezem, R.; van Loosdrecht, M.C. Syntrophic associations from hypersaline soda lakes converting organic acids and alcohols to methane at extremely haloalkaline conditions. Environ. Microbiol. 2016, 18, 3189–3202. [Google Scholar] [CrossRef]
- Sun, H.; Ni, P.; Angelidaki, I.; Dong, R.; Wu, S. Exploring stability indicators for efficient monitoring of anaerobic digestion of pig manure under perturbations. Waste Manag. 2019, 91, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Bize, A.; Bureau, C.; Bouchez, T.; Chapleur, O. Community shifts within anaerobic digestion microbiota facing phenol inhibition: Towards early warning microbial indicators? Water Res. 2016, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-F.; Xu, X.-H.; Dai, M.; Yuan, X.-Z.; Guo, R.-B. Hydrogen and methane production from vinasse using two-stage anaerobic digestion. Process Saf. Environ. Prot. 2017, 107, 81–86. [Google Scholar] [CrossRef]
- Lindner, J.; Zielonka, S.; Oechsner, H.; Lemmer, A. Is the continuous two-stage anaerobic digestion process well suited for all substrates? Bioresour. Technol. 2016, 200, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, R.; Hao, Y.; Yang, T.; Li, L.; Luo, Z.; Xie, L.; Zhao, N.; Liu, C.; Sun, C.; et al. CO2 and air pollutant emissions from bio-coal briquettes. Environ. Technol. Innov. 2023, 29, 102975. [Google Scholar] [CrossRef]
- Lloret, E.; Salar, M.J.; Blaya, J.; Pascual, J.A. Two-stage mesophilic anaerobic–thermophilic digestion for sludge sanitation to obtain advanced treated sludge. Chem. Eng. J. 2013, 230, 59–63. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sumardiono, S. Predicting kinetic model of biogas production and biodegradability organic materials: Biogas production from vinasse at variation of COD/N ratio. Bioresour. Technol. 2013, 149, 390–397. [Google Scholar] [CrossRef]
- Paranhos, A.G.O.; Adarme, O.F.H.; Barreto, G.F.; Silva, S.Q.; Aquino, S.F. Methane production by co-digestion of poultry manure and lignocellulosic biomass: Kinetic and energy assessment. Bioresour. Technol. 2020, 300, 122588. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy. Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Qiao, W.; Qiang, H.; Hojo, T.; Li, Y.Y. Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: Stability, inhibition and recovery. Bioresour. Technol. 2013, 137, 358–367. [Google Scholar] [CrossRef]
- Niu, Q.; Qiao, W.; Qiang, H.; Li, Y.Y. Microbial community shifts and biogas conversion computation during steady, inhibited and recovered stages of thermophilic methane fermentation on chicken manure with a wide variation of ammonia. Bioresour. Technol. 2013, 146, 223–233. [Google Scholar] [CrossRef]
- Siles, J.A.; Garcia-Garcia, I.; Martin, A.; Martin, M.A. Integrated ozonation and biomethanization treatments of vinasse derived from ethanol manufacturing. J. Hazard. Mater. 2011, 188, 247–253. [Google Scholar] [CrossRef]
- Nualsri, C.; Reungsang, A.; Plangklang, P. Biochemical hydrogen and methane potential of sugarcane syrup using a two-stage anaerobic fermentation process. Ind. Crop Prod. 2016, 82, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, R.; Massé, D.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Z.; Zhang, Y. Using straw as a bio-ethanol source to promote anaerobic digestion of waste activated sludge. Bioresour. Technol. 2019, 286, 121388. [Google Scholar] [CrossRef]
- España-Gamboa, E.I.; Mijangos-Cortés, J.O.; Hernández-Zárate, G.; Maldonado, J.A.D.; Alzate-Gaviria, L.M. Methane production by treating vinasses from hydrous ethanol using a modified UASB reactor. Biotechnol. Biofuels 2012, 5, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Cheng, H.; Chen, F.; Zhang, Y.; Xu, X.; Huang, C.; Chen, C.; Liu, W.; Ding, C.; Li, Z.; et al. Enhanced methane production by alleviating sulfide inhibition with a microbial electrolysis coupled anaerobic digestion reactor. Environ. Int. 2020, 136, 105503. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhen, G.; Ni, J.; Hojo, T.; Kubota, K.; Li, Y.Y. Effect of influent COD/SO42− ratios on biodegradation behaviors of starch wastewater in an upflow anaerobic sludge blanket (UASB) reactor. Bioresour. Technol. 2016, 214, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hao, Y.; Yang, T.; Wu, J.; Xu, F.; Li, L.; Wang, B.; Li, M.; Zhao, N.; Wang, N.; et al. Air pollutant emissions from sludge-bituminous briquettes as a potential household energy source. Case Stud. Therm. Eng. 2022, 37, 102251. [Google Scholar] [CrossRef]
- Hu, Y.; Jing, Z.; Sudo, Y.; Niu, Q.; Du, J.; Wu, J.; Li, Y.Y. Effect of influent COD/SO42− ratios on UASB treatment of a synthetic sulfate-containing wastewater. Chemosphere. 2015, 130, 24–33. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Kennedy, K.J.; Marin, J.; Strehler, B. Anaerobic digestion of whole stillage from dry-grind corn ethanol plant under mesophilic and thermophilic conditions. Bioresour. Technol. 2011, 102, 1079–1086. [Google Scholar] [CrossRef]
- Degueurce, A.; Tomas, N.; Le Roux, S.; Martinez, J.; Peu, P. Biotic and abiotic roles of leachate recirculation in batch mode solid-state anaerobic digestion of cattle manure. Bioresour. Technol. 2016, 200, 388–395. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Ahmed, P.M.; Nieto-Penalver, C.G.; de Figueroa, L.I.C.; Pajot, H.F. Vinasse odyssey: Sugarcane vinasse remediation and laccase production by Trametes sp. immobilized in polyurethane foam. Biodegradation 2022, 33, 333–348. [Google Scholar] [CrossRef]
- Raskin, L.; Stromley, J.M.; Rittmann, B.E.; Stahl, D.A. Group-Specific 16S rRNA Hybridization Probes To Describe Natural Communities of Methanogens. Appl. Environ. 1994, 60, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Galagan, J.E.; Nusbaum, C.; Roy, A.; Endrizzi, M.G.; Macdonald, P.; FitzHugh, W.; Calvo, S.; Engels, R.; Smirnov, S.; Atnoor, D.; et al. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 2002, 12, 532–542. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, S.; Solera, R.; Micolucci, F.; Cavinato, C.; Bolzonella, D. Changes in microbial community during hydrogen and methane production in two-stage thermophilic anaerobic co-digestion process from biowaste. Waste Manag. 2016, 49, 40–46. [Google Scholar] [CrossRef]
- Li, G.; Yang, T.; Xiao, W.; Wu, J.; Xu, F.; Li, L.; Gao, F.; Huang, Z. Sustainable environmental assessment of waste-to-energy practices: Co-pyrolysis of food waste and discarded meal boxes. Foods 2022, 11, 3840. [Google Scholar] [CrossRef]
- Zahedi, S.; Sales, D.; Romero, L.I.; Solera, R. Hydrogen production from the organic fraction of municipal solid waste in anaerobic thermophilic acidogenesis: Influence of organic loading rate and microbial content of the solid waste. Bioresour. Technol. 2013, 129, 85–91. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Schmidt, T.; Scholwin, F.; Il’inskaya, O.N.; Harms, H.; Kleinsteuber, S. Bacteria and archaea involved in anaerobic digestion of distillers grains with solubles. Appl. Microbiol. Biotechnol. 2011, 89, 2039–2052. [Google Scholar] [CrossRef]
- Li, J.; Zheng, G.; He, J.; Chang, S.; Qin, Z. Hydrogen-producing capability of anaerobic activated sludge in three types of fermentations in a continuous stirred-tank reactor. Biotechnol. Adv. 2009, 27, 573–577. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Liebetrau, J.; Proter, J.; Kleinsteuber, S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl. Microbiol. Biotechnol. 2013, 97, 5161–5174. [Google Scholar] [CrossRef]
- Sarti, A.; Pozzi, E.; Chinalia, F.A.; Ono, A.; Foresti, E. Microbial processes and bacterial populations associated to anaerobic treatment of sulfate-rich wastewater. Process. Biochem. 2010, 45, 164–170. [Google Scholar] [CrossRef]
- Yabuki, L.; Queluz, J.; Garcia, M. Assessment of phase distribution and removal of metals in anaerobic digesters. Int. J. Environ. Sci. Technol. 2022, 19, 463–474. [Google Scholar] [CrossRef]
- Wu, J.; Niu, Q.; Li, L.; Hu, Y.; Mribet, C.; Hojo, T.; Li, Y.Y. A gradual change between methanogenesis and sulfidogenesis during a long-term UASB treatment of sulfate-rich chemical wastewater. Sci. Total Environ. 2018, 636, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Mosbaek, F.; Kjeldal, H.; Mulat, D.G.; Albertsen, M.; Ward, A.J.; Feilberg, A.; Nielsen, J.L. Identification of syntrophic acetate-oxidizing bacteria in anaerobic digesters by combined protein-based stable isotope probing and metagenomics. ISME J. 2016, 10, 2405–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lv, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Fu, J.; Yan, B.; Gui, S.; Fu, Y.; Xia, S. Anaerobic co-digestion of thermo-alkaline pretreated microalgae and sewage sludge: Methane potential and microbial community. J. Environ. Sci. 2023, 127, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Parnaudeau, V.; Condom, N.; Oliver, R.; Cazevieille, P.; Recous, S. Vinasse organic matter quality and mineralization potential, as influenced by raw material, fermentation and concentration processes. Bioresour. Technol. 2008, 99, 1553–1562. [Google Scholar] [CrossRef]
- Wilk, M.; Krzywonos, M.; Seruga, P.; Walaszczyk, E. Effect of pH and temperature on vinasse decolorization by lactic acid bacteria in batch processes. Water Environ. Res. 2019, 91, 573–580. [Google Scholar] [CrossRef]
- Chandra, R.; Bharagava, R.N.; Rai, V. Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Bioresour. Technol. 2008, 99, 4648–4660. [Google Scholar] [CrossRef]
- Forouzanmehr, F.; Solon, K.; Maisonnave, V.; Daniel, O.; Volcke, E.I.P.; Gillot, S.; Buffiere, P. Sulfur transformations during two-stage anaerobic digestion and intermediate thermal hydrolysis. Sci. Total Environ. 2022, 810, 151247. [Google Scholar] [CrossRef]
- Welte, C.; Deppenmeier, U. Bioenergetics and anaerobic respiratory chains of aceticlastic methanogens. Biochim. Biophys. Acta 2014, 1837, 1130–1147. [Google Scholar] [CrossRef] [Green Version]
- Krzywonos, M.; Chałupniak, A.; Zabochnicka-Świątek, M. Decolorization of beet molasses vinasse by Bacillus megaterium ATCC 14581. Bioremediation J. 2017, 21, 81–88. [Google Scholar] [CrossRef]
- Tiwari, S.; Rai, P.; Yadav, S.K.; Gaur, R. A novel thermotolerant Pediococcus acidilactici B-25 strain for color, COD, and BOD reduction of distillery effluent for end use applications. Environ. Sci. Pollut. Res. Int. 2013, 20, 4046–4058. [Google Scholar] [CrossRef]
- Krayzelova, L.; Bartacek, J.; Kolesarova, N.; Jenicek, P. Microaeration for hydrogen sulfide removal in UASB reactor. Bioresour. Technol. 2014, 172, 297–302. [Google Scholar] [CrossRef]
- Shakeri Yekta, S.; Elreedy, A.; Liu, T.; Hedenstrom, M.; Isaksson, S.; Fujii, M.; Schnurer, A. Influence of cysteine, serine, sulfate, and sulfide on anaerobic conversion of unsaturated long-chain fatty acid, oleate, to methane. Sci. Total Environ. 2022, 817, 152967. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Li, Q.; Quan, X. Adding Fe0 powder to enhance the anaerobic conversion of propionate to acetate. Biochem. Eng. J. 2013, 73, 80–85. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Zhang, J.; Liang, S.; Ni, B.J.; Zhang, X.B.; Wang, J. Comparing the value of bioproducts from different stages of anaerobic membrane bioreactors. Bioresour. Technol. 2016, 214, 816–825. [Google Scholar] [CrossRef]
- De Sitter, K.; Garcia-Gonzalez, L.; Matassa, C.; Bertin, L.; De Wever, H. The use of membrane based reactive extraction for the recovery of carboxylic acids from thin stillage. Sep. Purif. Technol. 2018, 206, 177–185. [Google Scholar] [CrossRef]
- De Vrieze, J.; Gildemyn, S.; Arends, J.B.; Vanwonterghem, I.; Verbeken, K.; Boon, N.; Verstraete, W.; Tyson, G.W.; Hennebel, T.; Rabaey, K. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 2014, 54, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Cao, W.; Banks, C.J.; Heaven, S.; Liu, R. Biogas production from undiluted chicken manure and maize silage: A study of ammonia inhibition in high solids anaerobic digestion. Bioresour. Technol. 2016, 218, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lv, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Zhang, J.; Yip, C.; Xia, C.; Liang, Y. Evaluation of methane release from coals from the San Juan basin and Powder River basin. Fuel 2019, 244, 388–394. [Google Scholar] [CrossRef]
- Nallathambi, G. Anaerobic digestion of biomass for methane production: A review. Biomass Bioenergy 1997, 13, 83–114. [Google Scholar] [CrossRef]
- Yin, M.; Chen, H. Unveiling the dual faces of chitosan in anaerobic digestion of waste activated sludge. Bioresour. Technol. 2022, 344, 126182. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Wine | Sugar Beet | Sugarcane | Molasses | Cassava | Tequila |
|---|---|---|---|---|---|---|
| BOD (g/L) | - | - | - | 61.25 ± 0.56 | 12.25 ± 0.21 | 13–24 |
| TCOD (mg/L) | 40.22 ± 0.15 | 421.6 ± 26.5–541.9 ± 39.8 | 28.66 ± 0.91 | - | - | 28–50 |
| SCOD (mg/L) | 39.59 ± 0.09 | - | - | 134.10 ± 1023 | 38.210 ± 2810 | - |
| pH | 3.25 ± 0.14 | 4.96 ± 0.01–5.60 ± 0.02 | 4.03 ± 0.34 | 3.8 ± 0.6 | 4.3 ± 0.5 | 3.35 |
| TS (g/L) | 22.42 ± 0.09 | - | 25.00 ± 0.00 | 87.6 ± 7.6 | 60.5 ± 5.9 | 12 |
| VS (g/L) | 19.31 ± 0.08 | 422.5 ± 14.6–584.7 ± 13.7 | 18.00 ± 0.00 | 70.3 ± 6.2 | 49.4 ± 2.1 | 9.8 |
| C/N | 130.00 ± 3.18 | 5.9–6.0 | - | 53 | 21.3 | - |
| TAN (g/L) | 0.25 ± 0.04 | 1.07 ± 0.02–5.85 ± 0.10 | - | 0.19 ± 0.06 | - | - |
| TVFA (g/L) | 1.15 ± 0.04 | 16.70 ± 1.00–19.30 ± 1.10 | - | - | - | - |
| Acetic acid (g/L) | - | 16.30 ± 0.90–18.90 ± 1.00 | 0.66 | - | - | 2.5–3.4 |
| Propionic acid (g/L) | - | 0.13 ± 0.01–0.17 ± 0.01 | 1.70 | - | - | - |
| N (g/L) | - | - | 0.50 ± 0.95 | - | - | 0.24 |
| P (g/L) | - | 0.13 | 0.03 ± 0.00 | 1.42 ± 0.05 | 0.24 ± 0. 02 | 0.02 |
| Ref. | [13] | [33] | [38] | [39] | [39] | [40] |
| Research Purpose | Changed Operation Parameters | Conclusion | Ref. |
|---|---|---|---|
| Evaluate the effect of H2 partial pressure and acetic acid concentration on the carbon conversion of CO2 to methane and acetate for two different samples. | H2 partial pressure and acetic acid concentration | During hydrogenotrophic methanation for biogas upgrading it is essential to keep acetate levels below 0.8 g/L | [6] |
| Use a two-stage anaerobic system composed of two PBRs connected in series for the treatment of tequila vinasses under different OLRs. | OLR (g-COD L−1 d−1): 2.7 to 12.0 | 1. OLR:2.7 to 6.8 g-COD L−1 d−1, acetotrophic pathway as the main responsible for methane production 2. OLR > 12 g-COD L−1 d−1, hydrogenotrophic methane production pathway became dominant 3. High acetic acid concentrations (1.15 ± 0.2 g L−1) inhibit acetotrophic pathway | [16] |
| Clarify the effects of temperature and acetate concentration on the degradation of acetate and propionate. | temperature (35 and 55 °C) acetate concentration (20, 40, and 60 g-COD/L) | 1. High acetate concentration (60 g-COD/L), SAO is the dominant approach 2. SAO generally dominates over acetoclastic methanogenesis at high ammonia conditions | [25] |
| Discuss the influencing factors and potential mechanisms of hydrogen enrichment on phenol degradation and methane production. | ammonia concentrations and hydrogen partial pressure | The acetoclastic and hydrogenotrophic methanogenic activities were gradually improved with the increase of initial | [67] |
| Explore the mechanism of ammonium inhibition on AD of food waste. | different ammonium concentrations | With increasing ammonium concentration, from acetoclastic methanogenesis to SAO and hydrogenotrophic methanogenesis | [68] |
| Research Purpose | Less than the Threshold | Threshold | Continue to Rise | Maximum | Ref. |
|---|---|---|---|---|---|
| Effects of ammonia on AD of food waste. | Steady methane production | Ammonium: 2 g/L | Ammonium: 2 g/L to 4 g/L Methane production drops gradually and stops after a month | 5 g/L Strong inhibition | [68] |
| Effect of COD/N ratio on biogas production from distiller. | COD/N:400/7: the amount of Ammonium produced is too large, which can inhibit the activity of bacteria COD removal:33.483 ± 0.266% | COD/N: 600/7 | COD/N:700/7: Ammonium is not sufficient as a nitrogen source for the bacteria, and a small amount of biogas is eventually formed COD removal: 32.714 ± 0.881% | [84] | |
| Methane fermentation of chicken manure at different ammonia concentrations. | COD removal efficiency over 60% carbohydrate removal efficiency of 80% | Ammonium:5000 mg/L | Ammonium: 5000 mg/L to 15,000 mg/L VFAs accumulation (2000 to 15,000 mg/L) The removal efficiency of carbohydrate and protein decreased gradually. | 16,000 mg/L Production ceased | [87] |
| Methane fermentation of chicken manure at different ammonia concentrations. | VS high conversion rate High methane content (over 60%) pH and alkalinity are stable | Ammonium: 4000 mg/L | Ammonium: 4000 mg/L to 6000 mg/L VFAs accumulation (5000 to 25,000 mg/L) Methane production decreased from 0.20 L/g VS to 0.05 L/g VS. | 8000 mg/L Production almost ceased. | [88] |
| Bacterial Species | Optimum Condition | Other Findings | Maximum Decolorization Rate | Ref. |
|---|---|---|---|---|
| Trametes sp. | 30 °C, 21 days incubation, 2% glucose added in the implantation stage | Up to 1082 U L−1 laccase was obtained | 75% | [99] |
| Lactic acid bacteria (Lactobacillus plantarum, L. casei, and Pediococcus parvulus). | Non-controlled pH 6.5 and at 30°C | Controlled pH has a negative effect on sugar beet molasses vinasse decolorization | 25.14% | [117] |
| Bacillus megaterium ATCC 14581 | The decolorization efficiency of diluted distiller was not affected by the culture conditions of static and stirred bacteria culture | Glucose, ammonium sulfate, potassium dihydrogen phosphate, and distiller concentration are the most significant factors affecting distiller decolorization | 38% | [121] |
| Pediococcus acidilactici B-25 | 0.1% glucose at 45 °C | 1. The best carbon source: Glucose > Fructose, sucrose and starch > maltose and lactose 2. Peptone was found to be the best nitrogen source | 79% | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Xu, F.; Yang, T.; Wang, X.; Lyu, T.; Huang, Z. Microbial Behavior and Influencing Factors in the Anaerobic Digestion of Distiller: A Comprehensive Review. Fermentation 2023, 9, 199. https://doi.org/10.3390/fermentation9030199
Li G, Xu F, Yang T, Wang X, Lyu T, Huang Z. Microbial Behavior and Influencing Factors in the Anaerobic Digestion of Distiller: A Comprehensive Review. Fermentation. 2023; 9(3):199. https://doi.org/10.3390/fermentation9030199
Chicago/Turabian StyleLi, Gang, Fuzhuo Xu, Tenglun Yang, Xiqing Wang, Tao Lyu, and Zhigang Huang. 2023. "Microbial Behavior and Influencing Factors in the Anaerobic Digestion of Distiller: A Comprehensive Review" Fermentation 9, no. 3: 199. https://doi.org/10.3390/fermentation9030199