1. Introduction
Potato, a tuber originally from South America, is now one of the most important food crops in the world [
1]. It produces more dry matter and protein per hectare than other major cereal crops [
2]. More than 50% of the potatoes harvested in North America and Europe are processed to produce, e.g., French fries, chips, and starch [
1,
3]. Starch is a polysaccharide that is the main energy reserve of higher plants and has applications in food, feed and paper industries [
4]. According to the European Starch Industry Association, the starch market in Europe had a turnover of 8.3 billion € in 2014. Potato is the third most used raw material, accounting for 13.3% of starch production [
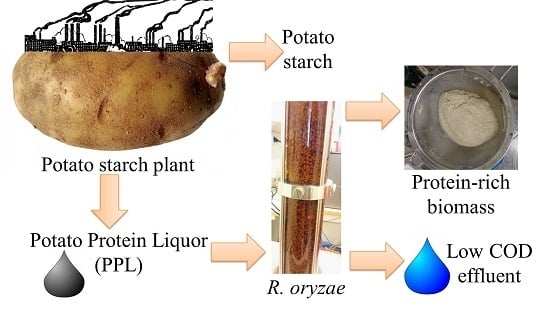
5]. Potato liquor, containing the soluble residues from potato starch production, is treated with steam at 110 °C to remove proteins. The remaining liquor is concentrated to potato protein liquor (PPL) and consists of approximately 40% solids [
6]. PPL is a severe challenge to wastewater treatment plants because of its high biological oxygen demand [
7]. Currently, the side stream is stocked during the year to be used as a fertilizer during planting. The strategy, however, requires large storage space in industrial facilities, contaminates ground water, and annoys adjacent areas with its bad odour. Additionally, PPL can be used as a supplement to cattle feed. However, it is not considered suitable because, despite its high nitrogen content, the quality of the proteins is low since they are oxidized during the steam treatment [
6,
8].
Alternative treatments have been suggested, attempting to find a destination for PPL, including using it as a substrate in different bioprocesses to produce yeast biomass [
9], fungal protein [
10], enzymes [
7], and other metabolites [
11]. However, the cultivation of filamentous fungi in this side stream has never been reported.
Filamentous fungi have been extensively used in several biotechnological applications to produce food, feed, and metabolites (e.g., enzymes, organic acids, antibiotics, cholesterol-lowering statins, and biofuels). They are commonly cultivated in submerged cultures, in which they can grow in three morphologies, namely, suspended mycelium, pellet, and clump. Some fungi are also able to grow in a yeast-like form. The morphology is influenced by the strain characteristics as well as the medium composition and cultivation conditions [
12,
13,
14,
15]. Growth in pellet form has been reported as a favourable alternative for fungal cultivation, because it enables repeated-batch fermentation and improves the rheology of the broth, resulting in better mass and oxygen transfer in the reactor with lower energy consumption [
12,
13,
14,
15]. Morphology of the fungi can affect the production of primary and secondary metabolites [
16].
Zygomycetes are receiving increased attention for both their capacity to produce a large variety of metabolic products and their valuable biomass, which contains several useful components, such as proteins, lipids, specific amino acids, chitosan, and chitin, making it eligible to be used for the production of animal feed, human food, and chitosan. Economic exploration of zygomycetous biomass is believed to be responsible for making the future establishment of a zygomycete-based biorefinery viable [
13,
17,
18,
19,
20,
21].
Rhizopus oryzae is one of the most widely used strains of zygomycetous fungi. Among the different bio-products produced by
R. oryzae, fumaric acid, lactic acid, pectinase, amyloglucosidase, α-amylase, and ethanol can be named [
12,
22]. Moreover, it can produce almost pure
l(+)-lactic acid without any rigorous nutritional requirements [
23]. Several investigations were performed to study the effect of culture medium on the morphology of
Rhizopus species and the relationship with biomass and metabolite productivity [
12,
16,
24,
25,
26,
27,
28,
29]. The use of
R. oryzae as fish feed has recently been noted as a potential replacement for fishmeal in fish feed composition because of the high protein content, comparable amino acid composition to fishmeal, and the proportion of C18 unsaturated fatty acids. In addition, several
R. oryzae strains are used in human food and are generally regarded as safe (GRAS) [
13]. Stirred-tank bioreactors are not common for the cultivation of filamentous fungi. On the other hand, airlift bioreactors are considered to be suitable choices for aerobic fungal cultivation, due to design simplicity, which favours increasing the scale and decreasing the processing costs and contamination risks [
30].
This study aimed to present a solution for the treatment of PPL and to use R. oryzae to reduce the organic load of potato starch side stream, while producing biomass, a potential product source. Firstly, PPL was characterized in terms of microorganism nutritional requirements such as carbohydrate and protein content. Secondly, the effect of liquor’s dilution on the morphology and yield of the fungal biomass was studied. At last, scaling up of the process to airlift reactors was performed at different aeration rates, aimed at obtaining the best conditions for pelleted fungal growth.
2. Materials and Methods
2.1. Microorganism
Rhizopus oryzae, CCUG 28958, was obtained from the Culture Collection of the University of Gothenburg (Gothenburg, Sweden). The strain was originally isolated from Indonesian food production (tempeh), and therefore, it is Generally Recognized As Safe (GRAS). The fungus was grown in 25-mL glass bottles for 5 days at 30 °C on a potato dextrose agar (PDA) medium containing (in g/L) potato extract 4; dextrose 20; agar 15. Then, the sporulated slants were stored at 5 °C (up to 30 days) until use. For inoculation, 12 mL of sterile tap water was poured on each slant. The slants were closed and manually agitated to release the spores. The spore suspension was then added to the liquid medium to reach a concentration between 2 × 105 and 5 × 105 spores/mL in the final medium.
2.2. Analytical Methods
d-Glucose, fructose, sucrose,
d-galactose, raffinose, starch,
l-asparagine,
l-glutamine, and ammonia were determined in liquid samples using the respective enzymatic assay kits from Megazyme (Bray, Ireland) following the company’s instructions. Liquid samples of PPL or the fermentation broth were centrifuged at 3000×
g for 10 min prior to analysis. When necessary, the samples were sufficiently diluted with distilled water to reach the concentration recommended in the kit. Kjeldahl nitrogen was determined using the InKjel P digestor and the Behrotest S1 distiller (Behr Labor-Technik, Dusseldorf, Germany). Acetic acid, lactic acid, glycerol, and ethanol, as the main metabolites, were analysed using high-performance liquid chromatography (HPLC) in a hydrogen-based ion-exchange column (Aminex HPX-87H, Bio-Rad, Hercules, CA, USA) at 60 °C using 0.6 mL/min of 0.5 mM H
2SO
4 solution as the eluent. The National Renewable Energy Laboratory (NREL) analytical procedure was used to determine total carbohydrates [
31] based on the hydrolysis of the polysaccharides using sulfuric acid and analysing the final solution on HPLC. A lead (II)-based column (Aminex HPX-87P, Bio-Rad) operating at 85 °C using 0.6 mL/min of ultrapure water as the eluent was used to detect the sugars that were released in the NREL procedure. Components were identified and quantified in the HPLC by a refractive index detector (Waters 2414, Milford, MA, USA).
The total solids were determined by drying a specified sample volume (10 mL) at 105 °C to a constant weight. The remaining solids were further placed in a muffle furnace (Gallenkamp, London, UK) at 550 °C to determine the ash content. The amount of insoluble solids was determined by centrifuging the PPL at 3000× g for 10 min and drying the obtained solid at the same condition that was previously mentioned. An AQUANAL™—professional tube test (1000–15,000 mg/L) (Sigma-Aldrich, St. Louis, MO, USA) was used to determine the chemical oxygen demand (COD) in liquid samples. All the waste generated from the experiments was sent to the appropriate treatment, following the Swedish guidelines. Residues of R. oryzae were sent to incineration.
2.3. Fungal Cultivation in Shake Flasks
PPL was obtained from Lyckeby Starch AB (Kristianstad, Sweden). All the experiments described in this paper used the same batch of liquor. R. oryzae was cultivated in 250 mL baffled Erlenmeyer flasks containing 50 mL of culture medium, composed of PPL diluted with tap water. Different PPL–water volume ratios (1:39, 1:19, 1:9, 1:4, 1:1.5) were examined. The medium was sterilized at 121 °C for 20 min before inoculation with 5 mL of the spore suspension at room temperature. The cultivation was carried out in a water bath shaker at 35 °C and 120 rpm for 48 h. The pH of the sample was not adjusted during the cultivation. Biomass morphologies and yields were observed in order to choose the best conditions for scaling up the cultivation to bench-scale airlift reactors. At the end of the cultivation, the fungal biomass was collected by passing the broth through a kitchen sieve, manually pressing the collected biomass, and then freeze-drying (0.3 mbar, condenser at −50 °C).
As a reference, cultivation of
R. oryzae in a synthetic medium containing either sucrose (9.5 g/L) or asparagine (9.5 g/L) as the carbon source was performed to test the consumption of these two substances. The medium also contained K
2HPO
4 (3.5 g/L), CaCl
2·2H
2O (1.0 g/L), MgSO
4·7H
2O (0.75 g/L), and (NH
4)
2SO
4 (7.5 g/L), and the pH was brought to 5.5 with 2 M H
2SO
4. After sterilization (121 °C, 20 min), the medium was supplemented with a vitamin solution (1 mL/L) and trace metals solution (10 mL/L) [
32] which were sterilized by filtration.
2.4. Fungal Cultivation on PPL in Airlift Reactor
Fungal cultivation was carried out in a 4-L airlift reactor (Belach Bioteknik AB, Skogås, Sweden) filled with 3.5 L of PPL–water solution. Inoculation was made using fungal pellets grown in PPL–water solution for 24 h in Erlenmeyer flasks under the same conditions as described in
Section 2.3. Cultivation lasted for 54 h with regular sampling in order to analyse the sucrose, glucose, fructose, asparagine, ammonia, ethanol, glycerol, acetic acid, and lactic acid. In order to improve the sucrose consumption, 1 mL of enzyme invertase (from yeast, in 50% glycerol, Megazyme, Ireland) was added to the reactor. The cultivation was carried out at 35 °C and the pH was controlled at 5.5 or below by addition of 2 M H
2SO
4. Biomass was harvested at the end of cultivation and freeze-dried. Different aeration rates were examined (0.57, 0.86, and 1.14 vvm) and the consumption of the substrate and the biomass yield were measured. Antifoam 204 (Sigma-Aldrich) was used to avoid foaming. The volume of antifoam needed was determined by aerating the medium before autoclaving at the same rate as it would be used in the test. The medium was sterilized inside the reactor, in autoclave, at 121 °C for 20 min.
2.5. Statistical Analysis
All the characterization tests and Erlenmeyer cultivations were performed in triplicate. The airlift cultivations were carried out in duplicate, and the presented results are the mean of the observed values and the standard errors of the means.
The software MINITAB® (version 17.1.0, Minitab Inc., State College, PA, USA) was used to statistically analyse the data collected from the experiments. Analyses of variance (ANOVA) were performed using general linear models and considering a confidence level of 95%.
3. Results
In this study, potato protein liquor (PPL), a by-product of the potato starch industry, was used for the cultivation of R. oryzae. PPL consists of a solution with the soluble residues of the potato starch production after the steam treatment to remove proteins. Its treatment and disposal is a challenge because of its high organic content. PPL was firstly characterized and then employed for preparation of fungal biomass. The yield and morphology of biomass as well as consumption of PPL ingredients during the course of fermentation were investigated.
3.1. PPL Characterization
PPL is described in the literature as a source of sugar and nitrogen for microorganisms [
6,
7,
9,
10]. The PPL used in this work was characterized by its concentration of sugars, nitrogen compounds, total solids, ashes, and COD. The results are presented in
Table 1. The pH was determined to be 5.35. The Total Kjeldahl Nitrogen (TKN) was above 20 g/L. As the main amino acid,
l-asparagine concentration was 20.98 ± 1.26 g/L. The density of the PPL was determined to be 1.15 g/mL.
Determination of total carbohydrates in the PPL by the NREL methodology showed a high carbohydrate content in this side stream. The total amount of glucose (including polysaccharides) was determined to be 72.63 ± 6.93 g/L PPL. Some glucose-containing compounds were determined by enzymatic kits (monomeric glucose, sucrose, raffinose and starch) which represent 62% of the total glucose measured in NREL methodology. Galactose amounted to 10.00 ± 2.43 g/L, and the concentration of arabinose was 1.30 ± 0.82 g/L. Mannose was not detected. Complete hydrolysis of sucrose, whose concentration was determined to be 47.43 ± 6.08 g/L, would yield 24.97 ± 3.20 g/L of glucose and the same amount of fructose. Total carbohydrates amounted to more than 120 g/L (considering total glucose, galactose and arabinose, and the determined forms of fructose).
3.2. Cultivation of Fungi on Different PPL Concentrations
As a first step,
R. oryzae was cultivated using PPL in Erlenmeyer flasks in order to determine the effect of its dilution on fungal growth. PPL was previously reported as containing all the nutrients necessary for microbial growth [
6,
7,
9,
10]. Therefore, no nutritional supplementation was used in any cultivation.
3.2.1. Biomass Yield
The yield of
R. oryzae biomass was affected by the degree of PPL dilution in the medium with water (
Table 2). Up to dilution 1:4, the increase in the PPL concentration resulted in an increase in the yield of biomass regarding the volume of PPL. A linear (
R2 = 0.998) relation of the biomass yield and the volume of PPL in the culture was obtained.
where
C stands for PPL concentration in the mixture (volume of PPL/volume of medium, e.g., for the 1:39 ratio,
C = 1/(39 + 1) = 0.025) and the biomass yield is given in grams of dry biomass per gram of carbohydrates.
3.2.2. Biomass Morphology
Increasing the PPL concentration in the mixture changed the
R. oryzae morphology from filaments to pellets and later to clumps. The different morphologies are shown in
Figure 1. When PPL was diluted by 1:39 ratio,
R. oryzae grew sparsely as filaments. At 1:19 dilution, more biomass was produced, resulting in entangled hyphae with a few small pellets. At 1:9 dilution, only pellets were obtained; and a dilution of 1:4 resulted in a large and single clump of the fungi. For each PPL–water solution, the fungus presented the same morphology since the beginning of its growth.
3.2.3. Metabolites Formation
Analysis of the metabolites in the medium after 48 h of cultivation of the fungus (
Table 3) showed an increase in the ammonia concentration. Glucose and asparagine concentrations decreased. At the end of the cultivation, raffinose, a trisaccharide composed of a glucose linked to a galactose and a fructose, disappeared and sucrose concentration increased. The pH went up to the alkaline region (between 7 and 8) in the diluted 1:39, 1:19, and 1:9 PPL:water media (
Table 2). Lactic acid, ethanol and glycerol were only formed at PPL–water ratio 1:1.5, reaching average concentrations of 2.21 ± 0.01, 0.31 ± 0.15, and 0.85 ± 0.06 g/L, respectively.
3.3. Sucrose and Asparagine Consumption
The results presented in
Table 3 indicate some challenges in the consumption of sucrose by the strain and the use of an amino acid as a carbon source. Asparagine, as well as glutamine, are the main free amino acids present in potato [
33]. Thus, an experiment was designed to investigate the role of these two compounds in
R. oryzae growth. Approximately 32% of the sucrose was hydrolysed to glucose and fructose during autoclavation as a result of the low pH of the medium (5.5).
Table 4 presents the concentration of the substances as well as the biomass collected and pH after 48 h of the cultivation. Less than 5% of the non-hydrolysed sucrose and 11.8% of the asparagine were consumed by the fungus after 48 h. The consumption of sugars decreased the pH to 2.8; while the asparagine consumption increased the pH to 7.2. The biomass grew as pellets in both cases. The biomass yield was 0.50 ± 0.07 g dry biomass/g of sugars consumed and 1.09 ± 0.04 g dry biomass/g of asparagine.
3.4. Fungal Cultivation in Airlift Bioreactors
As cultivation of the fungus at a PPL:water ratio of 1:9 resulted in fungal growth with pellet morphology as well as a high yield, this ratio was chosen for further investigation of the cultivations to airlift bioreactors. The aeration rate was chosen to vary from 0.57 to 1.14 vvm (volume of gas per culture medium per minute), i.e., 1 to 3 NL/min. Pellets were only obtained at the highest aeration rate examined, while at other aeration rates the fungus grew as clumps. The biomass yield increased from 41.63 ± 5.43 to 65.47 ± 2.91 g/L undiluted PPL when aeration increased from 0.57 to 1.14 vvm. Although high aeration was required and the medium content of the protein was high, no problems with foam were observed during the cultivation, provided that the antifoam had been added at the beginning of cultivation. The maximum antifoam volume that was necessary was 0.002 mL antifoam/mL medium.
The Kjeldahl nitrogen content of the biomass was also analysed, and the results are presented in
Table 5. The protein content can be estimated by multiplying the nitrogen content by 6.25 [
30]. Protein content is an important factor when fungal biomass is intended to be destined for feed applications. The maximum TKN was obtained at 0.86 vvm, representing 50% protein content (
w/
w).
During the first 24 h, the sugars were fully consumed. The sucrose was partially hydrolysed during the autoclavation of the medium. The remainder of this disaccharide was fully hydrolysed during the first 12 h of cultivation by the enzyme invertase added at the beginning of the cultivation. Asparagine was entirely consumed after 48 h, but the ammonium concentration, different from what was observed in shake flasks, did not increase (
Figure 2A–C). The pH levels were monitored and the profile is presented for the lower aeration case (
Figure 2D). The pH decreased from 5.4 in the beginning to 5.2 during the first day, together with the sugar consumption. When the sugar was finished, the pH started to increase and, after 46 h, it reached 5.5 and sulfuric acid started being added to the medium.
An increased aeration rate, as expected, reduced the ethanol production (
Figure 2D–F). In all of the cases, ethanol had a maximum concentration after 24 h. Lactic acid was also simultaneously produced. After the sugar was depleted, both ethanol and lactic acid were assimilated by the fungus. Glycerol was formed together with ethanol, reaching the highest concentration (1.13 ± 0.01 g/L) at 24 h in the lower aeration case and decreasing over a longer time. The value of the concentration of some components varied for each cultivation at time zero, e.g., lactic acid and glycerol. The difference may have been caused by e.g., the variable concentration of these components in the used inoculum. Different observed sucrose concentrations at time zero are the result of its hydrolysis during autoclavation as well as the action of the invertase enzyme in the period between the sampling and the sugar analysis. The acetic acid concentration, however, decreased from approximately 0.15 to 0 g/L during the first day (data not shown). This behaviour was expected because acetate is used in the process of aerobic respiration (e.g., for the formation of acetyl-CoA enzyme).
In all of the airlift bioreactor experiments in this work, the final COD of the medium was found to be 14.8 ± 0.1 g/L, against an initial value of 30 g COD/L (300 g/L of PPL 10-fold diluted). This represents more than a 50% reduction in COD, leading to a lower load for the wastewater plant and resulting in cost reduction in addition to biomass production, which can be used for animal feed.
4. Discussion
The PPL characterization results presented in
Table 1 are comparable with the literature; Schürgerl and Rosen reported a total sugar content between 4.0% and 14% (
w/
w) and a raw protein content between 16% and 24% (
w/
w) [
10]. The total sugar concentration of 8.7% (
w/
w) was in agreement with cited data, and Kjeldahl protein content (multiplying TKN by 6.25) of 11% (
w/
w) was found to be below that reported in literature. Davies showed asparagine and glutamine as the main free amino acids in potatoes [
33], while Heisler identified both amino acids in the composition of potato starch factory soluble waste [
34]. However, in the PPL sample used in this study,
l-glutamine was not found.
Using 1:4 water–diluted PPL for fungal cultivation resulted in 0.45 g of biomass/g of sugar consumed. Ferreira et al. obtained a maximum fungal biomass yield of 0.34 g/g of sugar using spent sulphite liquor [
30]. This might suggest either a lower inhibition in PPL, which results in lower consumption of the substrate for maintenance and can be further submitted to investigation, or the presence of other substances in the medium that could be used by the fungus as a substrate such as asparagine. Asparagine has long been considered to be a good source of carbon and nitrogen for microorganisms [
35]. Two enzymes were found to be involved in asparagine uptake by microorganisms: asparaginase, which catalyses the hydrolysis of asparagine to aspartate and ammonium, and aspartase ammonia-lyase, which catalyses the deamination of aspartate to fumarate and ammonium [
36]. The results of the experiments using asparagine as a substrate confirm the role of the asparagine as a carbon source and its contribution to a higher biomass yield.
The final pH in the alkaline region, together with the increased concentration of ammonia, contributed to stop the growth of
R. oryzae. The pH, however, did not increase in the cultivation with 1:4 PPL:water ratio due to the buffering capacity of the liquor. The raffinose can be hydrolysed to its monosaccharides by the enzymes α-galactosidase (acting in the glucose–galactose linkage) and β-fructosidase (cleaving the glucose–fructose bond) [
37]. The disappearance of raffinose with an increased sucrose concentration suggests the fungal ability to break the linkage between galactose and glucose but not the glucose–fructose linkage. The result is in agreement with Bulut et al., who have also described the poor metabolization of sucrose by
R. oryzae strain [
38].
In the cultivation using airlift bioreactors, the aeration rate showed an influence on fungal morphology. The fungal morphology during the microbial growth altered from clumps, when the tested aeration rates were 0.57 and 0.86 vvm, to pellets, when the aeration rate was 1.14 vvm. Increasing the aeration rate in the airlift bioreactors enlarges the superficial gas velocities, improving the volumetric mass transfer coefficient [
30], the gas holdup, and the liquid circulation velocity (shear force) [
39]. The cultivation showed a significantly better performance than that obtained in Erlenmeyer flasks (
Table 5) comparing the obtained biomass yields. For 0.86 and 1.14 vvm of aeration, the biomass yield in relation to the PPL used was significantly (
p < 0.025) higher than that obtained in shake flasks. The best condition tested was cultivation in a 1:9 diluted PPL medium and 1.14 vvm at 35 °C, given that these conditions resulted in pellet growth of the fungus. Sucrose was hydrolysed by the invertase enzyme added at the beginning of the cultivation. The glucose and fructose left in the medium after the cultivation represent 1.3% of the same sugars present at the beginning.
An interesting result from the airlift cultivation was that the ammonia concentration (in the form of ammonium salts) did not increase as it did in the Erlenmeyer flasks. One of the reasons for this different behaviour may be that, compared to the Erlenmeyer cultivation, more nitrogen was necessary to form more biomass with higher TKN concentration in the airlift reactor. Therefore, more ammonia was consumed. In
Section 3.3, it was determined that asparagine consumption results in a higher biomass yield than the one from sugar consumption. Thus, it may be an interesting strategy to wait for asparagine consumption in order to obtain more biomass.
Using
S. cerevisiae, Lotz et al. reduced the COD of a PPL solution by 44% (1:9 PPL–water ratio), from 32.2 to 18 g/L, using a continuously stirred-tank reactor [
9].
R. oryzae was able to reduce it to less than half of the initial values in the present study, proving its efficiency to reduce the organic load of the side stream while producing valuable biomass.