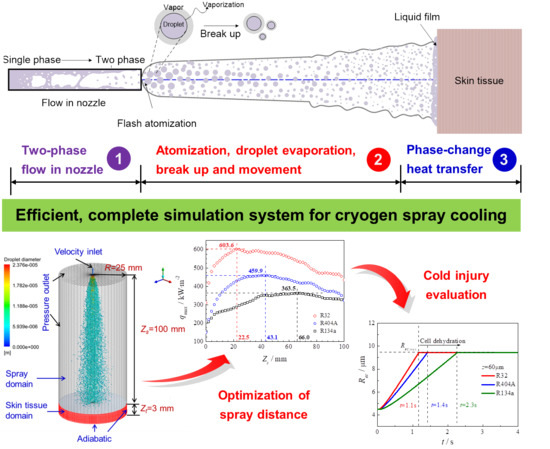
Theoretical Study on Cryogen Spray Cooling in Laser Treatment of Ota’s Nevus: Comparison and Optimization of R134a, R404A and R32
Abstract
:1. Introduction
2. Mathematical Modeling for Cryogen Spray Cooling
2.1. Discrete Phase Model for Spray Dynamics
2.1.1. Droplet Motion, Breakup and Evaporation
2.1.2. Governing Equations for Continuous Gas
2.2. Multi-Scale Heat Transfer Model for Skin Tissue Frostbite
2.3. Simulation Conditions and Model Validation
- (1)
- (2)
- (3)
- Calculate the gas concentration, velocity and temperature distribution by solving the mass, momentum and energy governing equations (Equations (5)–(7)) under the Eulerian framework with the implicit time scheme and SIMPLE algorithm;
- (4)
- Calculate the heat and mass transfer between the droplet and the surrounding gas using the two-way coupling method with the source terms Sm, F and Sh;
- (5)
- Calculate the heat flux on the skin surface with the surface heat transfer model (Equations (10) and (11)) [10];
- (6)
- Compute the temperature distribution by solving the macro-scale bio-heat conduction equation (Equation (8)) [31] coupled with the latent heat (L) released from the cellular water freezing;
- (7)
- Compute the probability of intracellular ice formation (PIIF) and the radius of the cylindrical extra-cellular space (Rec) by solving the micro-scale mass transfer equation (Equation (9)) [35].
3. Results and Discussions
3.1. Comparison of Spray Characteristics Using the Three Cryogens
3.2. Optimization of Spray Distance
3.3. Temperature Distribution Inside Skin Tissue during CSC
3.4. Optimization of the Interval between CSC and Laser Irradiation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | surface area (m2) |
| a | thermal diffusivity (m2·s−1) |
| Bm | Spalding mass transfer number |
| Bi* | spray Biot number, h*δ/λs |
| cp | specific heat (J·kg−1·K−1) |
| D | diameter (μm) |
| F | momentum source term (N·m−3·s−1) |
| Fos | Fourier number, ast/δ2 |
| g | acceleration of gravity (m·s−2) |
| h | convective heat transfer coefficient (W·m−2·K−1), enthalpy (J) |
| hfg | latent heat of droplet (J·kg−1) |
| J | diffusion rate (m2·s−1) |
| kc | mass transfer coefficient (m·s−1) |
| m | mass (kg) |
| p | pressure (Pa) |
| PIIF | probability of intracellular ice formation (%) |
| q | surface heat flux (kW·m−2) |
| Rec | radius (Rec) of extra-cellular space (μm) |
| Sh | energy source term (J·m−3·s−1) |
| Sm | mass source term (kg·m−3·s−1) |
| t | time (s) |
| T | temperature (°C) |
| u | velocity (m·s−1) |
| w | axial droplet velocity (m·s−1) |
| Z | axial distance (mm) |
| Greek symbols | |
| λ | heat conductivity coefficient (W·m−1·K−1), wavelength (nm) |
| ρ | density (kg·m−3) |
| σ | Stefan–Boltzmann constant (W·m−2·K−4), surface tension (N/m) |
| η | viscosity (Pa∙s) |
| δ | relative error (%) |
| µa | laser absorption coefficient (m−1) |
| ∆t | time interval between CSC and laser irradiation (ms) |
| ∆x | dimension of Krogh unit (μm) |
| stress tensor (Pa) | |
| Subscripts | |
| a | air |
| b | boiling point |
| c | cell |
| d | droplet |
| f | intracellular water |
| i | component |
| max | maximum value |
| ns | Krogh unit |
| r | radiation |
| s | spray |
| sat | saturation |
| t | skin tissue |
| 0 | initial value |
References
- Choi, J.E.; Lee, J.B.; Park, K.B.; Kim, B.S.; Yeo, U.C.; Huh, C.H.; Kim, J.H.; Kye, Y.C. A retrospective analysis of the clinical efficacies of Q-switched Alexandrite and Q-switched Nd:YAG lasers in the treatment of nevus of Ota in Korean patients. J. Dermatol. Treat. 2015, 26, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Suzuki, T. A New Classification of Ota’s Nevus Based on Histopathological Features. Dermatology 1991, 183, 169–172. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zou, Y.-L.; Zhang, L.; Liu, L. The Clinical and Histopathologicl Analysis of Zygomaticofacial Naevus Fuscoceruleus, Naevus of Ota and Melasma. Chin. J. Derm. 2003, 17, 25–29. [Google Scholar]
- Anderson, R.R.; Parrish, J.A. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science 1983, 220, 524–527. [Google Scholar] [CrossRef] [Green Version]
- MP, G. Cutaneous and Cosmetic Laser Surgery; Elsevier: Maryland Heights, MO, USA, 2006. [Google Scholar]
- Aguilar, G. Dynamics of cryogen deposition relative to heat extraction rate during cryogen spray cooling. Proc. SPIE 2000, 3907, 37–48. [Google Scholar] [CrossRef]
- Nelson, J.S.; Milner, T.E.; Anvari, B.; Tanenbaum, B.S.; Kimel, S.; Svaasand, L.O.; Jacques, S.L. Dynamic epidermal cooling during pulsed laser treatment of port-wine stain. A new methodology with preliminary clinical evaluation. Arch. Dermatol. 2016, 131, 695–700. [Google Scholar] [CrossRef]
- Dai, T.; Yaseen, M.A.; Diagaradjane, P.; Chang, D.W.; Anvari, B. Comparative study of cryogen spray cooling with R-134a and R-404a: Implications for laser treatment of dark human skin. J. Biomed. Opt. 2006, 11. [Google Scholar] [CrossRef]
- Zhou, Z.-F.; Chen, B.; Wang, R.; Wang, G.-X. Comparative investigation on the spray characteristics and heat transfer dynamics of pulsed spray cooling with volatile cryogens. Exp. Therm. Fluid Sci. 2017, 82, 189–197. [Google Scholar] [CrossRef]
- Tian, J.M.; Chen, B.; Li, D.; Zhou, Z.F. Transient spray cooling: Similarity of dynamic heat flux for different cryogens, nozzles and substrates. Int. J. Heat Mass Transf. 2017, 108, 561–571. [Google Scholar] [CrossRef]
- Dossat, R.J. Principles of Refrigeration; Prentice Hall: Englewood Cliffs, NJ, USA, 1991. [Google Scholar]
- No, E.J. Available online: http://www.ilo.org/dyn/cisdoc2/cismain.details?p_lang=en&p_doc_id=68628 (accessed on 28 May 2020).
- Aguilar, G.; Wang, G.X.; Nelson, J.S. Dynamic behavior of cryogen spray cooling: Effects of spurt duration and spray distance. Lasers Surg. Med. 2003, 32, 152–159. [Google Scholar] [CrossRef]
- Refrigerants—Designation and Safety Classification; ISO 817: 2014; ISO: Geneva, Switzerland, 2014.
- Aguilar, G.; Svaasand, L.O.; Nelson, J.S. Effects of hypobaric pressure on human skin: Feasibility study for port wine stain laser therapy (Part I). Lasers Surg. Med. 2005, 36, 124–129. [Google Scholar] [CrossRef]
- Norvang Nilsen, L.; Fiskerstrand, E.; Nelson, J.S.; Berns, M.; Svaasand, L. Epidermal Melanin Absorption in Human Skin; SPIE: Bellingham, WA, USA, 1996; Volume 2624. [Google Scholar]
- Wen-Lin, Y.U.; Dong, Z.; Qin, L.; Kai-Hua, Y.; Chun-Li, L. Virtual instrumentation technique and its application in skin spectrum detecting system. Chin. J. Aesthetic Med. 2007, 55, 34. [Google Scholar] [CrossRef]
- Chen, B.; Tian, J.; Wang, R.; Zhou, Z. Theoretical study of cryogen spray cooling with R134a, R404A and R1234yf: Comparison and clinical potential application. Appl. Therm. Eng. 2019, 148, 1058–1067. [Google Scholar] [CrossRef]
- Nilpueng, K.; Wongwises, S. Numerical simulation of refrigerants flowing through short-tube orifices during flashing process. Hvac R Res. 2013, 19, 159–174. [Google Scholar] [CrossRef]
- Zeng, Y.; Lee, C.-F. Modeling of Atomization Under Flash Boiling Conditions. J. Korean Soc. Combust. 2002, 7, 44–51. [Google Scholar]
- Witlox, H.; Harper, M.; Bowen, P.; Cleary, V. Flashing liquid jets and two-phase droplet dispersion: II. Comparison and validation of droplet size and rainout formulations. J. Hazard. Mater. 2007, 142, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Witlox, H.W.M.; Harper, M.; Oke, A.; Bowen, P.J.; Kay, P. Sub-cooled and flashing liquid jets and droplet dispersion I. Overview and model implementation/validation. J. Loss Prev. Process Indust. 2010, 23, 831–842. [Google Scholar] [CrossRef]
- Ashgriz, N. Handbook of Atomization and Sprays: Theory and Applications; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ranz, W.E.; Marshall, W.R. Evaporation from Droplets: Part I and II. Chem. Eng. Prog. 1952, 48, 141–173. [Google Scholar]
- Sirignano, W.A. Fluid Dynamics and Transport of Droplets and Sprays; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Zhou, Z.; Wang, G.; Chen, B.; Guo, L.; Wang, Y. Evaluation of Evaporation Models for Single Moving Droplet with a High Evaporation Rate. Powder Technol. 2013, 240, 95–102. [Google Scholar] [CrossRef]
- Taylor, G.I. The Shape and Acceleration of a Drop in a High Speed Air Stream. Sci. Pap. GI Taylor 1963, 3, 457–464. [Google Scholar]
- Reitz, R.D. Modeling atomization processes in high-pressure vaporizing sprays. At. Spray Technol. 1987, 3, 309–337. [Google Scholar] [CrossRef]
- Beale, J.C.; Reitz, R.D. Modeling Spray Atomization with the Kelvin-Helmholtz/Rayleigh Taylor Hybrid Model. At. Sprays 1999, 9, 623–650. [Google Scholar] [CrossRef]
- Apte, S.V.; Gorokhovski, M.; Moin, P. LES of atomizing spray with stochastic modeling of secondary breakup. Int. J. Multiph. Flow 2003, 29, 1503–1522. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.; Wu, W.J.; Wang, G.X.; He, Y.L. Multi-scale modeling of tissue freezing during cryogen spray cooling with R134a, R407c and R404a. Appl. Therm. Eng. 2014, 73, 1489–1500. [Google Scholar] [CrossRef]
- Morsi, S.A.; Alexander, A.J. An investigation of particle trajectories in two-phase flow systems. J. Fluid Mech. 2006, 55, 193–208. [Google Scholar] [CrossRef]
- Miller, R.S.; Harstad, K.; Bellan, J. Evaluation of equilibrium and non-equilibrium evaporation models for many-droplet gas-liquid flow simulations. Int. J. Multiph. Flow 1998, 24, 1025–1055. [Google Scholar] [CrossRef]
- Sazhin, S.S. Advanced models of fuel droplet heating and evaporation. Prog. Energy Combust. Sci. 2006, 32, 162–214. [Google Scholar] [CrossRef]
- Toner, M.; Cravalho, E.G.; Karel, M. Thermodynamics and kinetics of intracellular ice formation during freezing of biological cells. J. Appl. Phys. 1990, 67, 1582–1593. [Google Scholar] [CrossRef]
- Bischof, J.C.; Rubinsky, B. Microscale Heat and Mass Transfer of Vascular and Intracellular Freezing in the Liver. J. Heat Transf. 1993, 115, 1029–1035. [Google Scholar] [CrossRef]
- Kang, W.H.; Lee, E.S.; Choi, G.S. Treatment of Ota’s nevus by Q-switched alexandrite laser: Therapeutic outcome in relation to clinical and histopathological findings. Eur. J. Dermatol. EJD 2000, 9, 639–643. [Google Scholar]
- Zhou, Z.; Chen, B.; Wang, Y.; Guo, L.; Wang, G. An experimental study on pulsed spray cooling with refrigerant R-404a in laser surgery. Appl. Therm. Eng. 2012, 39, 29–36. [Google Scholar] [CrossRef]
- Aguilar, G.; Díaz, S.H.; Lavernia, E.J.; Nelson, J.S. Cryogen spray cooling efficiency: Improvement of port wine stain laser therapy through multiple-intermittent cryogen spurts and laser pulses. Lasers Surg. Med. 2002, 31, 27–35. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, W.; Chen, B.; Wang, G.; Guo, L. An experimental study on the spray and thermal characteristics of R134a two-phase flashing spray. Int. J. Heat Mass Transf. 2012, 55, 4460–4468. [Google Scholar] [CrossRef]


















| Cryogens | ρd (kg/m3) | cp,d (J/kg∙K) | λd (W/m∙K) | Tb (°C) | psat (MPa) | σ (N/m) | η (Pa∙s) | hfg (J/kg) |
|---|---|---|---|---|---|---|---|---|
| R134a | 1206.7 | 1424.6 | 0.0811 | −26.1 | 0.67 | 0.0081 | 0.00019 | 216,000 |
| R404A | 1044.1 | 1542.3 | 0.0646 | −46.5 | 1.25 | 0.0046 | 0.00013 | 230,260 |
| R32 | 961.0 | 1936.7 | 0.1259 | −51.9 | 1.69 | 0.0068 | 0.00011 | 270,910 |
| Properties | States | Epidermis | Dermis |
|---|---|---|---|
| ρ (kg·m−3) | Unfrozen | 1120 | 1090 |
| Completely frozen | 1120 | 921 | |
| c (J·kg−1·K−1) | Unfrozen | 3200 | 3500 |
| Completely frozen | 3200 | 1230 | |
| λ (W·m−1·K−1) | Unfrozen | 0.34 | 0.41 |
| Completely frozen | 0.34 | 2.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Chen, B.; Zhou, Z.; Li, D. Theoretical Study on Cryogen Spray Cooling in Laser Treatment of Ota’s Nevus: Comparison and Optimization of R134a, R404A and R32. Energies 2020, 13, 5647. https://doi.org/10.3390/en13215647
Tian J, Chen B, Zhou Z, Li D. Theoretical Study on Cryogen Spray Cooling in Laser Treatment of Ota’s Nevus: Comparison and Optimization of R134a, R404A and R32. Energies. 2020; 13(21):5647. https://doi.org/10.3390/en13215647
Chicago/Turabian StyleTian, Jiameng, Bin Chen, Zhifu Zhou, and Dong Li. 2020. "Theoretical Study on Cryogen Spray Cooling in Laser Treatment of Ota’s Nevus: Comparison and Optimization of R134a, R404A and R32" Energies 13, no. 21: 5647. https://doi.org/10.3390/en13215647