Consortium Growth of Filamentous Fungi and Microalgae: Evaluation of Different Cultivation Strategies to Optimize Cell Harvesting and Lipid Accumulation
Abstract
:1. Introduction
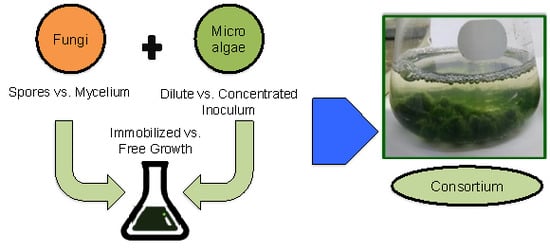
2. Materials and Methods
2.1. Microorganisms: Maintenance, Culture Medium, and Growth
2.2. Analytical Methods
2.3. Calculation of Biochemical Parameters
3. Results
3.1. Axenic and Combined Cell Growth
3.2. Evaluation of Different Strategies for Higher Algae Recovery Efficiencies
3.3. Effects on Lipid Content and Fatty Acid Distribution
4. Discussion
4.1. Evaluation of the Culture Medium and Simultaneous Inoculation of Algae Cells and Fungal Spores
4.2. Other Strategies Involving the Use of Mature Fungal Mycelium
4.3. The Effect of Different Strategies on the Lipid Accumulation and Properties
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Henderson, R.K.; Parsons, S.A.; Jefferson, B. Successful removal of algae through the control of zeta potential. Sep. Sci. Technol. 2008, 43, 1653–1666. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Pragya, N.; Pandey, K.K.; Sahoo, P.K. A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew. Sustain. Energy Rev. 2013, 24, 159–171. [Google Scholar] [CrossRef]
- Gultom, S.; Hu, B. Review of microalgae harvesting via co-pelletization with filamentous fungus. Energies 2013, 6, 5921–5939. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hu, B. A novel method to harvest microalgae via co-culture of filamentous fungi to form cell pellets. Bioresour. Technol. 2012, 114, 529–535. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Bento, H.B.S.; Carvalho, A.K.F.; Rajendran, A.; Hu, B.; De Castro, H.F. Critical applications of Mucor circinelloides within a biorefinery context. Crit. Rev. Biotechnol. 2019, 39, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.G.; Mc Bride, J.E.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.K.F.; Rivaldi, J.D.; Barbosa, J.C.; De Castro, H.F. Biosynthesis, characterization and enzymatic transesterification of single cell oil of Mucor circinelloides—A sustainable pathway for biofuel production. Bioresour. Technol. 2015, 181, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Loures, C.C.A.; Amaral, M.S.; Da Rós, P.C.M.; Zorn, S.M.F.E.; De Castro, H.F.; Silva, M.B. Simultaneous esterification and transesterification of microbial oil from Chlorella minutissima by acid catalysis route: A comparison between homogeneous and heterogeneous catalysts. Fuel 2018, 211, 261–268. [Google Scholar] [CrossRef]
- Rajendran, A.; Hu, B. Mycoalgae biofilm: Development of a novel platform technology using algae and fungal cultures. Biotechnol. Biofuels 2016, 9, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, M.; Gilles, A.K.; Hamilton, K.J.; Rebers, A.P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Liang, F.; Jin, F.; Liu, H.; Wang, Y.; Chang, F. The molecular function of the yeast polo-like kinase Cdc5 in Cdc14 release during early anaphase. Mol. Biol. Cell 2009, 20, 3671–3679. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar] [CrossRef]
- Shong, J.; Diaz, M.R.J.; Collins, C.H. Towards synthetic microbial consortia for bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 798–802. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Szeghalmi, A.; Kaminskyj, S.; Gough, K.M. A synchrotron FTIR microspectroscopy investigation of fungal hyphae grown under optimal and stressed conditions. Anal. Bioanal. Chem. 2007, 387, 1779–1789. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef]
- Barnharst, T.; Rajendran, A.; Hu, B. Bioremediation of synthetic intensive aquaculture wastewater by a novel feed-grade composite biofilm. Int. Biodeterior. Biodegrad. 2018, 126, 131–142. [Google Scholar] [CrossRef]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PLoS ONE 2014, 9, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, H.; Wang, Q. A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 2019, 275, 35–43. [Google Scholar] [CrossRef]
- Du, Y.Z.; Alvaro, J.; Hyden, B.; Zienkiewicz, K.; Benning, N.; Zienkiewicz, A.; Bonito, G.; Benning, C. Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol. Biofuels 2018, 11, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, A.; Fox, T.; Hu, B. Nutrient recovery from ethanol co-products by a novel mycoalgae biofilm: Attached cultures of symbiotic fungi and algae. J. Chem. Technol. Biotechnol 2017, 92, 1766–1776. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, J.; Zhang, W.; Hu, B. A new cultivation method for microbial oil production: Cell pelletization and lipid accumulation by Mucor circinelloides. Biotechnol. Biofuels 2011, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, D.T.; Yeh, K.L.; Chen, C.L.; Chang, J.S. Enzymatic transesterification of microalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol. 2012, 108, 119–127. [Google Scholar] [CrossRef]
- Zamalloa, C.; Gultom, S.O.; Rajendran, A.; Hu, B. Ionic effects on microalgae harvest via microalgae-fungi co-pelletization. Biocatal. Agric. Biotechnol. 2017, 9, 145–155. [Google Scholar] [CrossRef]
- Rajendran, A.; Fox, T.; Reis, C.R.; Wilson, B.; Hu, B. Deposition of manure nutrients in a novel mycoalgae biofilm for Nutrient management. Biocatal. Agric. Biotechnol. 2018, 14, 120–128. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Rajendran, A.; Silva, M.B.; Hu, B.; De Castro, H.F. The application of microbial consortia in a biorefinery context: Understanding the importance of artificial lichens. In Sustainable Biotechnology-Enzymatic Resources of Renewable Energy; Singh, O., Chandel, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 423–437. [Google Scholar] [CrossRef]
- Talebi, A.F.; Mohtashami, S.K.; Tabatabaei, M.; Tohidfar, M.; Bagheri, A.; Zeinalabedini, M.; Mirzaei, H.H.; Mirzajanzadeh, M.; Shafaroudi, S.M.; Bakhtiari, S. Fatty acids profiling: A selective criterion for screening microalgae strains for biodiesel production. Algal Res. 2013, 2, 258–267. [Google Scholar] [CrossRef]
- Wynn, J.P.; Hamid, A.A.; Li, Y.; Ratledge, C. Biochemical events leading to the diversion of carbon into storage lipids in the oleaginous fungi Mucor circinelloides and Mortierella alpina. Microbiology 2001, 147, 2857–2864. [Google Scholar] [CrossRef] [Green Version]
- Hultberg, M.; Jönsson, H.L.; Bergstrand, K.J.; Carlsson, A.S. Impact of light quality on biomass production and fatty acid content in the microalga Chlorella vulgaris. Bioresour. Technol. 2014, 159, 465–467. [Google Scholar] [CrossRef]






| Culture Condition | Total Lipids (% Dry Biomass Weight) | Total Lipids (mg·L−1) | QL (mg·L−1·day−1) |
|---|---|---|---|
| Axenic photoautotrophic C. vulgaris | 23.8 ± 1.0 | 190.4 ± 1.0 | 25.4 ± 1.0 |
| Axenic M. circinelloides | 7.1 ± 0.2 | 63.9 ± 0.8 | 8.5 ± 0.8 |
| Strategy 1 | 22.7 ± 0.4 | 272.4 ± 0.5 | 36.3 ± 0.5 |
| Strategy 2 | 23.5 ± 0.8 | 253.8 ± 1.0 | 33.8 ± 0.5 |
| Strategy 3 | 19.0 ± 0.5 | 190.0 ± 0.5 | 22.0 ± 0.5 |
| Strategy 4 | 22.2 ± 0.4 | 230.8 ± 0.5 | 30.8 ± 0.5 |
| Parameter | Culture Time (Days) | ||||
|---|---|---|---|---|---|
| 8 | 10 | 12 | 14 | 16 | |
| Consortium Biomass (mg·L−1) | 1037 ± 7 | 806 ± 8 | 932 ± 7 | 1025 ± 7 | 1150 ± 6 |
| Microalgae Recovery (%) | 99.4 ± 0.2 | 98.8 ± 0.2 | 98.5 ± 0.2 | 98.3 ± 0.2 | 98.0 ± 0.2 |
| Total Lipids (% of Dry Biomass) | 31.1 ± 0.5 | 32.8 ± 0.5 | 31.6 ± 0.5 | 30.1 ± 0.5 | 30.5 ± 0.5 |
| Fatty Acids | Weight contribution to the total Fatty Acids (%) | ||||
| C 12:0 | 1.25 | 1.12 | 1.27 | 1.11 | 1.01 |
| C 14:0 | 0.91 | 0.87 | 0.90 | 0.76 | 0.71 |
| C 15:0 | 6.79 | 6.67 | 6.78 | 7.27 | 7.06 |
| C 16:0 | 26.58 | 26.79 | 25.56 | 23.81 | 26.25 |
| C 17:0 | 1.70 | 1.62 | 1.28 | 2.68 | 2.22 |
| C 18:0 | 1.31 | 1.29 | 1.63 | 1.79 | 2.03 |
| C 16:1 | 0.91 | 0.92 | 0.86 | 0.87 | 0.97 |
| C 18:1 | 32.98 | 33.06 | 35.0 | 31.34 | 30.07 |
| C 18:2 | 11.81 | 11.72 | 12.14 | 11.74 | 11.55 |
| C 18:3 | 15.76 | 15.93 | 14.57 | 18.64 | 18.13 |
| Fungal strain | Microalgae Strain | Culture Medium | Immobilization Matrix | Microalgae Recovery (%) | Consortium Biomass (mg·L−1) | Microalgae: Fungal Weight Contribution to the Consortium | Reference |
|---|---|---|---|---|---|---|---|
| Mortierella isabellina, Fusarium equiseti, F. lacertarum, Nigrospora oryzae, Altermaria alternate, F.equiseti, M.hiemalis, and M. circinelloides | C. vulgaris UTEX 2714 | Medium A | Polypropylene spun and tape yarn | 34.85 ± 8.1–99.94 ± 0.02 (strain specific) | 611.6 ± 11.9–1243.0 ± 37 (strain specific) | 4.9: 95.1–48.9:51:0 | Rajendran and Hu [10] |
| M. indicus ATCC 24905 | C. vulgaris UTEX 2714 | Medium A supplemented with ammonium concentrations ranging from 0 to 100 mg L−1 | Polypropylene spun and tape yarn | 61.26–97.70 ([NH4+]-specific) | 657.2–1125.0 ([NH4+]-specific) | 19.72:80.28–80:99-19.01 ([NH4+]-specific) | Barnharst et al. [18] |
| A.fumigatus | C. vulgaris, Chlamydomonas reinhardtii, Pseudokirchneriella subcapitata,Scenedesmus quadricauda, Thraustochytrid sp, Dunaliella tertiolecta, D.salina, Nannochloropsis oculata, Tetraselmis chuii, and Pyrocystis lunula | Fungal growth broth with glucose or acid-pre-treated wheat straw and various dilutions of anaerobically digested swine wastewater | Self-fungal pelletization | ≅ 25 -> 95 (strain and medium specific) | 110–1060 (strain and medium specific) | Not specified | Wrede et al. [19] |
| Aspergillus sp. | C. vulgaris | Pre-treated molasses | Not Disclosed | >95 | Up to 4125 (condition specific) | 26.3:73.7–95.2:4.8 (condition specific) | Yang et al. [20] |
| M.elongata AG 77 | N.oceanica CCMP 1779 | Guillard F/2 medium | Not Disclosed | >60 | Approximately 1000 | Not specified | Du et al. [21] |
| M. circinelloides URM 4182 | C. vulgaris BMAK D07 | Medium A | Self-fungal pelletization | 99.5 ± 0.2 | 1023 ± 27 | 79 ± 0.4: 21 ± 0.4 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorn, S.M.F.E.; Reis, C.E.R.; Silva, M.B.; Hu, B.; De Castro, H.F. Consortium Growth of Filamentous Fungi and Microalgae: Evaluation of Different Cultivation Strategies to Optimize Cell Harvesting and Lipid Accumulation. Energies 2020, 13, 3648. https://doi.org/10.3390/en13143648
Zorn SMFE, Reis CER, Silva MB, Hu B, De Castro HF. Consortium Growth of Filamentous Fungi and Microalgae: Evaluation of Different Cultivation Strategies to Optimize Cell Harvesting and Lipid Accumulation. Energies. 2020; 13(14):3648. https://doi.org/10.3390/en13143648
Chicago/Turabian StyleZorn, Savienne M. F. E., Cristiano E. R. Reis, Messias B. Silva, Bo Hu, and Heizir F. De Castro. 2020. "Consortium Growth of Filamentous Fungi and Microalgae: Evaluation of Different Cultivation Strategies to Optimize Cell Harvesting and Lipid Accumulation" Energies 13, no. 14: 3648. https://doi.org/10.3390/en13143648