Study on the Possibilities of Natural Use of Ash Granulate Obtained from the Combustion of Pellets from Plant Biomass
Abstract
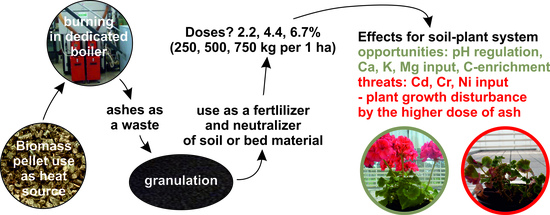
:1. Introduction
2. Materials and Methods
2.1. Biomass Preparation and Analysis
2.2. Preparation of Granulate from Ash
- pure ash,
- 955 g ash + 45 g bentonite,
- 910 g ash + 90 g bentonite,
- 775 g ash + 225 g bentonite.
2.3. Studies on the Possible Use of Ash
- a Venlo type greenhouse; cultivation in pots on mobile tables; the building is equipped with a climate computer made by the company Synopta (TANAKE, Warsaw, Poland), HortiMax (Hortisystems, Pulborough, West Sussex, England), MultiMa (TANAKE, Warsaw, Poland), using the following parameters: precipitation, radiation intensity, wind speed, temperature outside and inside the greenhouse, humidity for controlling the mechanisms of the greenhouse;
- a cultivated field located in the Centre, near plastic tents; cultivation in soil—Brunic Arenosols (Aric), according to the World reference base for soil resources 2014, update 2015.
- 450 g substrate without ash (A);
- 440 g substrate + 10 g ash granulate (B); ash share in the mixture: 2.22%;
- 430 g substrate + 20 g ash granulate (C); ash share in the mixture: 4.44%;
- 420 g substrate + 30 g ash granulate (D); ash share in the mixture: 6.66%.
2.4. Methodology of Laboratory Analyses
- Grain-size composition was determined using the sieve method and the hydrometric method, as specified in the description of the Polish Society of Soil Science, Classification of the granulation of soils and mineral structures, from 2008;
- pH was determined potentiometrically in a mixture of air-dry solid material: water 1:5, using an InoLab pH meter equipped with a WTW SenTix 41 glass electrode (Xylem Analytics, Weilheim Germany);
- total carbon content (TC), inorganic carbon content (IC) and total organic carbon content (TOC)—using the Pregla–Dumas method—dry samples were combusted in a pure oxygen environment and the resulting exhaust gases were automatically measured using a CHNS/O 2400 Series II PerkinElmer elemental analyser (PerkinElmer Inc., Baesweiler, Germany). The measurements were conducted for weights of 1.5–2.5 mg in three repetitions, from which the mean and standard deviations were calculated;
- specific conductivity (EC) was determined conductometrically in mixture of air-dry solid material:water 1:5; using a Eutech Instruments Cyberscan PC300 (Thermo Fisher Scientific Inc., Waltham, MA, USA) and an Elmetron CPC-411 with an EC-60 conductivity sensor (Elmetron, Zabrze, Poland);
- carbonate content was determined using a standardized method consistent with ISO 10693;
- subtotal content of selected components was determined after the dissolution of hot substrate samples (in a Perkin Elmer MPS microwave oven) in a mixture of hydrochloric and nitric acids (Aqua regia), using the ICP-OES method (Perkin Elmer Optima 8000)—ISO 11466 (1995); simultaneously, in the Perkin Elmer laboratory, pure materials—bentonite and ash were analysed for comparison;
- potential availability of components for plants in acidic substrate were analysed in an extract of 0.1 M HCl. The extract was prepared as cold by mixing a sample of the substrate with an extractor for 1 h in a soil mixer. The analysis was carried out using the ICP-OES method (Perkin Elmer Optima 8000) by Page et al. [41].
2.5. Analysis of the Results
3. Results and Discussion
3.1. Characteristics of Biomass Pellets
3.2. Analysis of Ash Properties
3.3. Analysis of the Chemical Composition of the Substrates
3.4. Analysis of the Chamical Composition of the Plants
- in the greenhouse:
- increased uptake of Cd and Cr by the begonias and the thujas and Cu by the begonias in comparison to the standard content of these elements in plants;
- Cd content tolerable in agronomic crops exceeded six times in the roots of the thujas (3.18 mg·kg−1);
- in the field:
- increased uptake of Cd and Cu by the roots and Ni by the aboveground parts of the begonias in comparison to the standard content of these elements in plants;
- Cr content tolerable in agronomic crops exceeded 20 times in the aboveground parts of the begonias (41.37 mg·kg−1);
- increased uptake of Ni by the thujas (in the roots) and Cu (in the aboveground parts) and Cd and Cr in all parts of the plants in comparison to the standard content of these elements in plants;
- Cr content tolerable in agronomic crops exceeded in all parts of the pelargoniums (3–9.11 mg·kg−1);
- Cu content tolerable in agronomic crops exceeded in the aboveground parts of the thujas (68.3 mg·kg−1), and the Cd and Cr threshold exceeded in the roots (respectively 3.52 mg·kg−1 and 4.49 mg·kg−1).
- in the greenhouse:
- increased uptake of Cd by the roots and Cr by all parts of the begonias, and Cr by the aboveground parts of the thujas in comparison to the standard content of these elements in plants;
- Cd content tolerable in agronomic crops exceeded over seven times in aboveground parts of the thujas (3.79 mg·kg−1);
- in the field:
- increased uptake of Cd and Cu by the roots of the begonias in comparison to the standard content of these elements in plants;
- increased uptake of Cd and Cr by the thujas in all parts of the plants in comparison to the standard content of these elements in plants;
- Cr content tolerable in agronomic crops exceeded in all parts of the pelargoniums (2.85–10 mg·kg−1);
- Cr content tolerable in agronomic crops exceeded seven times in the roots of the begonias (3.79 mg·kg−1) and 14 times in the aboveground parts of these plants (7.47 mg·kg−1);
- Cu content tolerable in agronomic crops exceeded three times (60.8 mg·kg−1), and the Cd content tolerable in agronomic crops exceeded two times in the roots of the begonias (0.98 mg·kg−1);
- Cd and Cr content tolerable in agronomic crops exceeded in the roots of the thujas (respectively 3.35 mg·kg−1 and 2.5 mg·kg−1).
- in the greenhouse:
- increased uptake of Cr by the shoots and leaves of the geraniums, the roots of the begonias and the roots of the thujas in comparison to the standard content of these elements in plants;
- increased uptake of Cd by all parts of the plant;
- Cd content tolerable in agronomic crops exceeded in all parts of the begonias (0.7–0.98 mg·kg−1);
- Cd content tolerable in agronomic crops exceeded 11 times in the roots of the thujas (5.51 mg·kg−1);
- in the field:
- increased uptake of Cd and Cu by the roots, Cr by all parts and Ni in the aboveground parts of the begonias in comparison to the standard content of these elements in plants;
- increased uptake of Cr by the thujas in all parts and Cd in the roots in comparison to the standard content of these elements in plants;
- Cr content tolerable in agronomic crops exceeded in all parts of the pelargoniums (5.97–20.6 mg·kg−1);
- Cr content tolerable in agronomic crops exceeded in all parts of the begonias, including 11 times in the aboveground parts (55.27 mg·kg−1), the Cd content tolerable in agronomic crops slightly exceeded in the roots (0.56 mg·kg−1), the Cu content tolerable in agronomic crops exceeded almost four times in the roots (77.4 mg·kg−1), and the Ni content tolerable in agronomic crops exceeded 2.5 times in the aboveground parts (24.9 mg·kg−1);
- Cd content tolerable in agronomic crops exceeded and the Cr content tolerable in agronomic crops exceeded six times in the roots of the thujas (2.99 mg·kg−1).
- relation between Ca in the substrate and in the biomass of the bedding pelargoniums (correlation coefficient r = −0.97) and the begonias (correlation coefficient r = 0.97),
- relation between Mg in the substrate and in the biomass of the begonias (correlation coefficient r = 0.95),
- relation between Ni in the substrate and in the biomass of the bedding pelargoniums (correlation coefficient r = 0.99).
4. Conclusions and Perspectives for Further Research
- A significant content of total carbon in the ash was noted, especially in its course fraction. For this reason, fertilization with ash may increase the carbon stock in soils and affect the soil sorption of elements.
- Ash from biomass combustion is a good neutralizer of soil and horticultural substrate. It is also a material rich in Ca, K and Mg.
- High bioavailability of Pb and Cd from ash as well as the relatively high availability of Zn are an environmental problem. The mobility of these heavy metals was significantly reduced by the admixture of bentonite during ash granulation. However, with high doses of ash pellets the uptake of Cd by the crop plants was high. We found it as a negative aspect, strongly affecting the possibility of the agricultural use of ash, counting the need to ensure ecological safety of such technology.
- During cultivation, Cr was activated, which results in the presence of high concentrations of this element in the plants cultivated on both the greenhouse substrates as well as on the soil in the field. We found it as another negative aspect, resulting from the use of ash as a fertilizer.
- Preparation of ash granulate with an admixture of bentonite in the proportion: 225 g bentonite and 775 g ash increased the sorption of components in this material and decreased their bioavailability but a significant release of heavy metals to the substrate was still noted. In the long run, this might be a significant problem if soil fertilized in this way is used for the cultivation of long-rotation plants.
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Y.; Jensen, P.A.; Jensen, A.D.; Sander, B.; Junker, H. Ash transformation during co-firing coal and straw. Fuel 2006, 86, 1008–1020. [Google Scholar] [CrossRef]
- Cuellar, A.D.; Herzog, H. A Path Forward for Low Carbon Power from Biomass. Energies 2015, 8, 1701–1715. [Google Scholar] [CrossRef] [Green Version]
- Łączak, A.; Bazan-Krzywoszańska, A.; Mrówczyńska, M.; Skiba, M. Renewable energy sources in the Lubusz Voivodship (Poland). The present conditions and perspectives for development. Civ. Environ. Eng. Rep. 2018, 28, 31–67. [Google Scholar] [CrossRef]
- Rybak, A. Poland’s Energy Mix and Energy Security of the Country. World Sci. News 2019, 128, 402–415. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- James, A.K.; Thring, R.W.; Helle, S.; Ghuman, H.S. Ash Management Review—Applications of Biomass Bottom Ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef]
- García-Maraver, A.; Popov, V.; Zamorano, M. A review of European standards for pellet quality. Renew. Energy 2011, 36, 3537–3540. [Google Scholar] [CrossRef]
- Houshfar, E.; Løvås, T.; Skreiberg, Ø. Experimental Investigation on NOx Reduction by Primary Measures in Biomass Combustion: Straw, Peat, Sewage Sludge, Forest Residues and Wood Pellets. Energies 2012, 5, 270–290. [Google Scholar] [CrossRef] [Green Version]
- Tchapda, A.H.; Pisupati, S.V. A Review of Thermal Co-Conversion of Coal and Biomass/Waste. Energies 2014, 7, 1098–1148. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Wang, J.; Preto, F.; Zhu, J.; Xu, C. Ash Deposition in Biomass Combustion or Co-Firing for Power/Heat Generation. Energies 2012, 5, 5171–5189. [Google Scholar] [CrossRef]
- European Union Institutions, Bodies, Offices and Agencies. EU 2018/C 124/01 Commission Notice on Technical Guidance on the Classification of Waste. Off. J. Eur. Union 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF (accessed on 9 April 2018).
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Falemara, B.C.; Joshua, V.I.; Aina, O.O.; Nuhu, R.D. Performance Evaluation of the Physical and Combustion Properties of Briquettes Produced from Agro-Wastes and Wood Residues. Recycling 2018, 3, 37. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Combustion of Miscanthus: Composition of the Ash by Particle Size. Energies 2019, 12, 178. [Google Scholar] [CrossRef]
- Huinink, J.T.M. Soil quality requirements for use in urban environments. Soil Tillage Res. 1998, 47, 157–162. [Google Scholar] [CrossRef]
- Saletnik, B.; Zagula, G.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biochar and Biomass Ash as a Soil Ameliorant: The Effect on Selected Soil Properties and Yield of Giant Miscanthus (Miscanthus x giganteus). Energies 2018, 11, 2535. [Google Scholar] [CrossRef]
- Cabrera, M.; Galvin, A.P.; Agrela, F.; Beltrán, M.; Ayuso, J. Reduction of Leaching Impacts by Applying Biomass Bottom Ash and Recycled Mixed Aggregates in Structural Layers of Roads. Materials 2016, 9, 228. [Google Scholar] [CrossRef]
- Nurmesniemi, H.; Manskinen, K.; Poykio, R.; Dahl, O. Forest fertilizer properties of the bottom ash and fly ash from a large-sized (115 MW) industrial power plant incinerating wood-based biomass residues. J. Univ. Chem. Technol. Metall. 2012, 47, 43–52. [Google Scholar]
- Antonkiewicz, J.; Pełka, R. Fractions of heavy metals in soil after the application of municipal sewage sludge, peat, and furnace ash. Soil Sci. Ann. 2014, 65, 118–125. [Google Scholar] [CrossRef]
- Gibczyńska, M.; Stankowski, S.; Hury, G.; Kuglarz, K. Effects of limestone, ash from biomass and compost use on chemical properties of soil. Soil Sci. Ann. 2014, 65, 59–64. [Google Scholar] [CrossRef]
- Tejasvi, A.; Kumar, S. Impact of Fly Ash on Soil Properties. Natl. Acad. Sci. Lett. 2012, 35, 13–16. [Google Scholar] [CrossRef]
- Ćwiąkała, M.; Greinert, A.; Kostecki, J.; Rafalski, L. The possibility to use modified flight ash as a neutralizer in the acid soils reclamation processes. Civ. Environ. Eng. Rep. 2018, 28, 88–104. [Google Scholar] [CrossRef]
- Hansen, H.K.; Pedersen, A.J.; Ottosen, L.M.; Villumsen, A. Speciation and mobility of cadmium in straw and wood combustion fly ash. Chemosphere 2001, 45, 123–128. [Google Scholar] [CrossRef]
- Lima, A.T.; Ottosen, L.M.; Ribeiro, A.B. Assessing fly ash treatment: Remediation and stabilization of heavy metals. J. Environ. Manag. 2012, 95, S110–S115. [Google Scholar] [CrossRef] [PubMed]
- Stankowski, S.; Sobolewska, M.; Jaroszewska, A.; Gibczyńska, M. Influence of biomass ash, lime and gypsum fertilization on macro- and microelement contents in the soil and grains of spring wheat. Soil Sci. Ann. 2018, 69, 177–183. [Google Scholar] [CrossRef]
- Bakisgan, C.; Dumanli, A.G.; Yürüm, Y. Trace elements in Turkish biomass fuels: Ashes of wheat straw, olive bagasse and hazelnut shell. Fuel 2009, 88, 1842–1851. [Google Scholar] [CrossRef] [Green Version]
- Greinert, A. Clays as substances limiting phytotoxic influence of Pb, Zn and Cd in sandy soils. In Contaminated Soil’95; Van Den Brink, W.J., Bosman, R., Arendt, F., Eds.; Springer: Dordrecht, The Netherlands, 1995; Volume 5, pp. 1223–1225. ISBN 978-94-011-0421-0. [Google Scholar]
- Greinert, A. Improving the sorption properties of soils as obligatory for maintaining green urban areas in good condition. Soil Sci. Ann. 2009, 60, 75–83. [Google Scholar]
- Zha, F.; Liu, S.; Du, Y.; Cui, K. Behavior of expansive soils stabilized with fly ash. Nat. Hazards 2008, 47, 509–523. [Google Scholar] [CrossRef]
- Kuo, W.T.; Gao, Z.C. Engineering Properties of Controlled Low-Strength Materials Containing Bottom Ash of Municipal Solid Waste Incinerator and Water Filter Silt. Appl. Sci. 2018, 8, 1377. [Google Scholar] [CrossRef]
- Papandreou, A.D.; Stournaras, C.J.; Panias, D.; Paspaliaris, I. Adsorption of Pb(II), Zn(II) and Cr(III) on coal fly ash porous pellets. Miner. Eng. 2011, 24, 1495–1501. [Google Scholar] [CrossRef]
- Backes, C.A.; Pulford, I.D.; Duncan, H.J. Seasonal Variation of Pyrite Oxidation Rates in Colliery Spoil. Soil Use Manag. 1993, 9, 30–34. [Google Scholar] [CrossRef]
- Meyer, G.; Waschkies, C.; Hüttl, R.F. Investigations on pyrite oxidation in mine spoils of the Lusatian lignite mining district. Plant Soil 1999, 213, 137–147. [Google Scholar] [CrossRef]
- Drab, M.; Greinert, A. Calcium content in post-mining grounds in the Łęknica Region. Civ. Environ. Eng. Rep. 2010, 4, 46–57. [Google Scholar]
- Dung, T.T.T.; Vassilieva, E.; Swennen, R.; Cappuyns, V. Release of Trace Elements from Bottom Ash from Hazardous Waste Incinerators. Recycling 2018, 3, 36. [Google Scholar] [CrossRef]
- Yu, Q.; Sawayama, S.; Sugita, S.; Shoya, M.; Isojima, Y. The reaction between rice husk ash and Ca(OH)2 solution and the nature of its product. Cem. Concr. Res. 1999, 29, 37–43. [Google Scholar] [CrossRef]
- Martirena, F.; Middendorf, B.; Day, R.L.; Gehrke, M.; Roque, P.; Martinez, L. Rudimentary, low tech incinerators as a means to produce reactive pozzolan out of sugar cane straw. Cem. Concr. Res. 2006, 36, 1056–1061. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Jia, S.; Richardson, I.G. Micro- and nano-structural evolutions in white Portland cement/pulverized fuel ash cement pastes due to deionized-water leaching. Cem. Concr. Res. 2018, 103, 191–203. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Mehrali, M.; Alengaram, U.J.; Jumaat, M.Z. Graphene nanoplatelet-fly ash based geopolymer composites. Cem. Concr. Res. 2015, 76, 222–231. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbiological Methods; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1982; pp. 323–336. ISBN 0-89118-072-9. [Google Scholar]
- Toscano, G.; Alfano, V.; Scarfone, A.; Pari, L. Pelleting Vineyard Pruning at Low Cost with a Mobile Technology. Energies 2018, 11, 2477. [Google Scholar] [CrossRef]
- Ribbing, C. Environmentally friendly use of non-coal ashes in Sweden. Waste Manag. 2007, 27, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Smriti, S.; Ram, L.C.; Masto, R.E.; Verma, S.K. A comparative evaluation of minerals and trace elements in the ashes from lignite, coal refuse, and biomass fired power plants. Int. J. Coal Geol. 2011, 87, 112–120. [Google Scholar] [CrossRef]
- Karapinar, N.; Donat, R. Adsorption behaviour of Cu2+ and Cd2+ onto natural bentonite. Desalination 2009, 249, 123–129. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. A Case Study of Landfill Leachate Using Coal Bottom Ash for the Removal of Cd2+, Zn2+ and Ni2+. Metals 2016, 6, 300. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Biogeochemia Pierwiastków Śladowych; PWN: Warszawa, Poland, 1999; ISBN 3-01128-23-2. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press LLC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Lis-Krzyścin, A. N Fertilization and Nutrient Status of Geranium Plant Pelargonium x hortium. Acta Agrophys. 2006, 7, 651–661. [Google Scholar]
- Grigatti, M.; Giorgioni, M.E.; Ciavatta, C. Compost-based growing media: Influence on growth and nutrient use of bedding plants. Bioresour. Technol. 2007, 98, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Cregg, B. Conifer nutrition. The Michigan Landscape. Conifer Corner 2005, 9–10, 42–45. [Google Scholar]
- Kuang, Y.W.; Wen, D.Z.; Zhou, G.Y.; Liu, S.Z. Distribution of elements in needles of Pinus massoniana (Lamb.) was uneven and affected by needle age. Environ. Pollut. 2007, 145, 146–153. [Google Scholar] [CrossRef] [PubMed]









| Substrate Composition | Plant Used in the Experiment | Combination Code |
|---|---|---|
| A; base substrate (control) | R1 Pelargonium x hortorum L. H. Bailey “Salomon Pink”) | AR1 |
| B; substrate + 10 g ash granulate | BR1 | |
| C; substrate + 20 g ash granulate | CR1 | |
| D; substrate + 30 g ash granulate | DR1 | |
| A; base substrate (control) | R2 Begonia semperflorens cult. hort. “Olimpia” | AR2 |
| B; substrate + 10 g ash granulate | BR2 | |
| C; substrate + 20 g ash granulate | CR2 | |
| D; substrate + 30 g ash granulate | DR2 | |
| A; base substrate (control) | R3 Thuja occidentalis L. | AR3 |
| B; substrate + 10 g ash granulate | BR3 | |
| C; substrate + 20 g ash granulate | CR3 | |
| D; substrate + 30 g ash granulate | DR3 |
| Ash | C | S | N | H | O | Cl | K | Ca |
|---|---|---|---|---|---|---|---|---|
| 2.74 | 51.64 | 0.14 | 0.28 | 6.30 | 41.64 | 0.21 | 0.542 | 0.246 |
| Material Analysed | Sieve Analysis (%) | Hydrometric Analysis (%) | Material | |||
|---|---|---|---|---|---|---|
| <2 mm | >2 mm | 2–0.05 mm | 0.05–0.002 mm | <0.002 mm | ||
| Ash | 82.8 | 17.2 | 85 | 15 | 0 | Sand (S) |
| Bentonite | 100.0 | 0.0 | 23 | 52 | 25 | Silt loam (SiL) |
| Ash + bentonite | 51.3 | 48.7 | 73 | 22 | 5 | Loamy sand (LS) |
| Material Analysed | Carbon Content (%) | pH–H2O | EC | CaCO3 | ||
|---|---|---|---|---|---|---|
| TOC | IC | TC | mS·cm−1 | % | ||
| Ash; fraction < 2 mm | 13.55 | 0.45 | 14.00 | 10.3 | 8.13 | 4.54 |
| Ash; fraction > 2 mm | 21.19 | 0.11 | 21.30 | 10.2 | 8.13 | 4.54 |
| Bentonite | 2.29 | 0.70 | 2.99 | 8.9 | 10.00 | 7.95 |
| Ash (fraction < 2 mm) + bentonite | 11.30 | 0.50 | 11.80 | 9.1 | 9.70 | 6.81 |
| (% d.m.) | |||||||
|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Na | Si | Al | Fe |
| 0.16 | 7.19 | 12.90 | 1.75 | 0.45 | 26.51 | 0.64 | 0.79 |
| (mg·kg−1 d.m.) | |||||||
| Cl | Cd | Cr | Cu | Mn | Ni | Pb | Zn |
| <100 | 2.17 | 230 | 66.4 | 3840 | 81.4 | 0.77 | 236 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greinert, A.; Mrówczyńska, M.; Szefner, W. Study on the Possibilities of Natural Use of Ash Granulate Obtained from the Combustion of Pellets from Plant Biomass. Energies 2019, 12, 2569. https://doi.org/10.3390/en12132569
Greinert A, Mrówczyńska M, Szefner W. Study on the Possibilities of Natural Use of Ash Granulate Obtained from the Combustion of Pellets from Plant Biomass. Energies. 2019; 12(13):2569. https://doi.org/10.3390/en12132569
Chicago/Turabian StyleGreinert, Andrzej, Maria Mrówczyńska, and Wojciech Szefner. 2019. "Study on the Possibilities of Natural Use of Ash Granulate Obtained from the Combustion of Pellets from Plant Biomass" Energies 12, no. 13: 2569. https://doi.org/10.3390/en12132569