Contribution of the Collective Excitations to the Coupled Proton and Energy Transport along Mitochondrial Cristae Membrane in Oxidative Phosphorylation System
Abstract
:1. Introduction
2. Model Background and Formulation
2.1. The OXPHOS System Organization in Mitochondrial Inner Membrane Folds
2.2. Role of Cardiolipin in Cristae Membrane Structure, Dynamics and Function
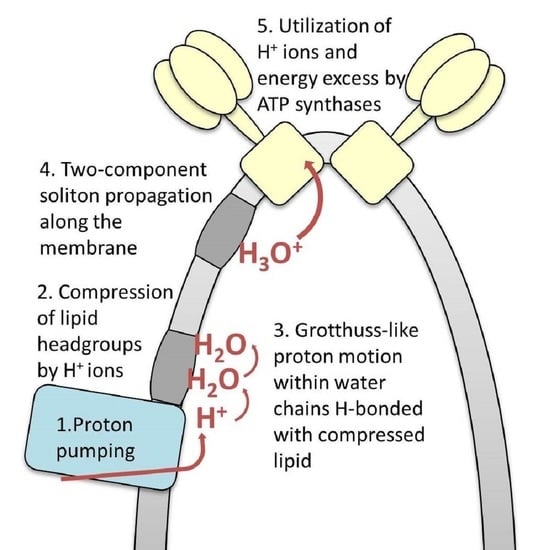
2.3. Grotthuss-Like Mechanism of Proton Transfer along the HB Chains
2.4. A Two-Component Model of Lateral Proton Transport along Mitochondria Cristae Membrane
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulkidjanian, A.Y.; Heberle, J.; Cherepanov, D.A. Protons @ interfaces: Implications for biological energy conversion. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 913–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesterov, S.V.; Smirnova, E.G.; Yaguzhinsky, L.S. Mechanism of Energy Storage and Transformation in the Mitochondria at the Water–Membrane Interface. Biochemistry 2022, 87, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Kovbasnjuk, O.N.; Yaguzhinsky, L.S. Evidence in favor of the existence of a kinetic barrier for proton transfer from a surface of bilayer phospholipid membrane to bulk water. Biochim. Biophys. Acta (BBA)-Biomembr. 1993, 1150, 45–50. [Google Scholar] [CrossRef]
- Tashkin, V.Y.; Vishnyakova, V.E.; Shcherbakov, A.A.; Finogenova, O.A.; Ermakov, Y.A.; Sokolov, V.S. Changes of the Capacitance and Boundary Potential of a Bilayer Lipid Membrane Associated with a Fast Release of Protons on Its Surface. Biochem. Suppl. Ser. A Membr. Cell Biol. 2019, 13, 155–160. [Google Scholar] [CrossRef]
- Sjöholm, J.; Bergstrand, J.; Nilsson, T.; Šachl, R.; Von Ballmoos, C.; Widengren, J.; Brzezinski, P. The lateral distance between a proton pump and ATP synthase determines the ATP-synthesis rate. Sci. Rep. 2017, 7, 2926. [Google Scholar] [CrossRef] [Green Version]
- Yaguzhinsky, L.S.; Boguslavsky, L.I.; Volkov, A.G.; Rakhmaninova, A.B. Synthesis of ATP coupled with action of membrane protonic pumps at the octane–water interface. Nature 1976, 259, 494–496. [Google Scholar] [CrossRef]
- Weichselbaum, E.; Österbauer, M.; Knyazev, D.; Batishchev, O.V.; Akimov, S.; Nguyen, T.H.; Zhang, C.; Knör, G.; Agmon, N.; Carloni, P.; et al. Origin of proton affinity to membrane/water interfaces. Sci. Rep. 2017, 7, 453. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W. Mitochondrial energetics with transmembrane electrostatically localized protons: Do we have a thermotrophic feature? Sci. Rep. 2021, 11, 14575. [Google Scholar] [CrossRef]
- Medvedev, E.; Stuchebrukhov, A.A. Mechanism of long-range proton translocation along biological membranes. FEBS Lett. 2012, 587, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Cherepanov, D.A.; Junge, W.; Mulkidjanian, A.Y. Proton Transfer Dynamics at the Membrane/Water Interface: Dependence on the Fixed and Mobile pH Buffers, on the Size and Form of Membrane Particles, and on the Interfacial Potential Barrier. Biophys. J. 2004, 86, 665–680. [Google Scholar] [CrossRef]
- Nath, S. Supercomplex Supercomplexes: Raison d’etre and Functional Significance of Supramolecular Organization in Oxidative Phosphorylation. Biomol. Concepts 2022, 13, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Junge, W.; Ausländer, W. The Electric Generator in Photosynthesis of Green Plants. I. Vectorial and Protolytic Properties of the Electron Transport Chain. Biochim. Biophys. Acta (BBA)-Bioenerg. 1974, 333, 59–70. [Google Scholar] [CrossRef]
- Drachev, L.A.; Kaulen, A.D.; Skulachev, V.P. Correlation of Photochemical Cycle, H+ Release and Uptake, and Electric Events in Bacteriorhodopsin. FEBS Lett. 1984, 178, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Heberle, J.; Riesle, J.; Thiedemann, G.; Oesterhelt, D.; Dencher, N.A. Proton Migration along the Membrane Surface and Retarded Surface to Bulk Transfer. Nature 1994, 370, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, V.S.; Motovilov, K.A.; Lobysheva, N.V.; Orlov, V.N.; Yaguzhinsky, L.S. The Formation of Metastable Bond between Protons and Mitoplast Surface. Dokl. Biochem. Biophys. 2011, 438, 127–130. [Google Scholar] [CrossRef]
- Toth, A.; Meyrat, A.; Stoldt, S.; Santiago, R.; Wenzel, D.; Jakobs, S.; von Ballmoos, C.; Ott, M. Kinetic Coupling of the Respiratory Chain with ATP Synthase, but Not Proton Gradients, Drives ATP Production in Cristae Membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 2412–2421. [Google Scholar] [CrossRef] [Green Version]
- Rieger, B.; Junge, W.; Busch, K.B. Lateral PH Gradient between OXPHOS Complex IV and F0F1 ATP-Synthase in Folded Mitochondrial Membranes. Nat. Commun. 2014, 5, 3103. [Google Scholar] [CrossRef] [Green Version]
- Morelli, A.M.; Ravera, S.; Calzia, D.; Panfoli, I. An Update of the Chemiosmotic Theory as Suggested by Possible Proton Currents inside the Coupling Membrane. Open Biol. 2019, 9, 180221. [Google Scholar] [CrossRef] [Green Version]
- Deplazes, E.; Poger, D.; Cornell, B.; Cranfield, C.G. The Effect of H3O+ on the Membrane Morphology and Hydrogen Bonding of a Phospholipid Bilayer. Biophys. Rev. 2018, 10, 1371–1376. [Google Scholar] [CrossRef]
- Marx, D. Proton Transfer 200 Years after von Grotthuss: Insights from Ab Initio Simulations. ChemPhysChem 2006, 7, 1848–1870. [Google Scholar] [CrossRef]
- Wraight, C.A. Chance and Design—Proton Transfer in Water, Channels and Bioenergetic Proteins. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 886–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCoursey, T. Voltage-Gated Proton Channels and Other Proton Transfer Pathways. Physiol. Rev. 2003, 83, 475–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakashita, N.; Ishikita, H.; Saito, K. Rigidly Hydrogen-Bonded Water Molecules Facilitate Proton Transfer in Photosystem II. Phys. Chem. Chem. Phys. 2020, 22, 15831–15841. [Google Scholar] [CrossRef] [PubMed]
- Borshchevskiy, V.; Kovalev, K.; Round, E.; Efremov, R.; Astashkin, R.; Bourenkov, G.; Bratanov, D.; Balandin, T.; Chizhov, I.; Baeken, C.; et al. True-Atomic-Resolution Insights into the Structure and Functional Role of Linear Chains and Low-Barrier Hydrogen Bonds in Proteins. Nat. Struct. Mol. Biol. 2022, 29, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Hagen, V.; Cherepanov, D.A.; Antonenko, Y.N.; Pohl, P. Protons Migrate along Interfacial Water without Significant Contributions from Jumps between Ionizable Groups on the Membrane Surface. Proc. Natl. Acad. Sci. USA 2011, 108, 14461–14466. [Google Scholar] [CrossRef] [Green Version]
- Antonchenko, B.Y.; Davydov, A.S.; Zolotaryuk, A.V. Solitons and Proton Motion in Ice-like Structures. Phys. Status Solidi B 1983, 115, 631. [Google Scholar] [CrossRef]
- Davydov, A.S. Solitons in Quasi-One-Dimensional Molecular Structures. Sov. Phys. Usp. 1982, 25, 898. [Google Scholar] [CrossRef]
- Austin, R.H.; Xie, A.; van der Meer, L.; Shinn, M.; Neil, G. Self-Trapped States in Proteins? J. Phys. Condens. Matter 2003, 15, S1693–S1698. [Google Scholar] [CrossRef]
- Pang, X. The Properties of Bio-Energy Transport and Influence of Structure Nonuniformity and Temperature of Systems on Energy Transport along Polypeptide Chains. Prog. Biophys. Mol. Biol. 2012, 108, 1–46. [Google Scholar] [CrossRef]
- Bolterauer, H.; Tuszyński, J.A.; Satarić, M.V. Fröhlich and Davydov Regimes in the Dynamics of Dipolar Oscillations of Biological Membranes. Phys. Rev. A 1991, 44, 1366–1381. [Google Scholar] [CrossRef]
- Kadantsev, V.N.; Goltsov, A. Collective Excitations in α-Helical Protein Structures Interacting with the Water Environment. Electromagn. Biol. Med. 2020, 39, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, L.; Priya, R.; Ayyappan, N.; Gopi, D.; Jayanthi, S. Energy Transport Mechanism in the Form of Proton Soliton in a One-Dimensional Hydrogen-Bonded Polypeptide Chain. J. Biol. Phys. 2016, 42, 9–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manousakis, E. Collective Charge Excitations along Cell Membranes. Phys. Lett. A 2005, 342, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Kadantsev, V.N.; Goltsov, A.N. Collective dynamics of domain structures in liquid crystalline lipid bilayers. Russ. Technol. J. 2022, 10, 44–54. [Google Scholar] [CrossRef]
- Matsui, H.; Matsuo, Y. Proton Conduction via Water Bridges Hydrated in the Collagen Film. J. Funct. Biomater. 2020, 11, 61. [Google Scholar] [CrossRef]
- Davydov, A.S. Solitons in Molecular Systems; Mathematics and Its Applications; Springer: Dordrecht, The Netherlands, 1985; Volume 4, ISBN 978-94-017-3027-3. [Google Scholar]
- Davydov, A.S. Solitons and Energy Transfer along Protein Molecules. J. Theor. Biol. 1977, 66, 379–387. [Google Scholar] [CrossRef]
- Savin, A.V.; Zolotaryuk, A.V. Two-Sublattice Solitons in Hydrogen-Bonded Chains with Dynamical Disorder. In Nonlinearity with Disorder; Abdullaev, F., Bishop, A.R., Pnevmatikos, S., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 238–251. [Google Scholar]
- Zolotaryuk, A.V.; Peyrard, M.; Spatschek, K.H. Collective Proton Transport with Weak Proton-Proton Coupling. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 62, 5706–5710. [Google Scholar] [CrossRef] [Green Version]
- Lupichev, L.N.; Savin, A.V.; Kadantsev, V.N. Synergetics of Molecular Systems; Springer Series in Synergetics; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-08194-6. [Google Scholar]
- Golovnev, A.; Eikerling, M. Theory of Collective Proton Motion at Interfaces with Densely Packed Protogenic Surface Groups. J. Phys. Condens. Matter 2012, 25, 045010. [Google Scholar] [CrossRef]
- Nesterov, S.; Chesnokov, Y.; Kamyshinsky, R.; Panteleeva, A.; Lyamzaev, K.; Vasilov, R.; Yaguzhinsky, L. Ordered Clusters of the Complete Oxidative Phosphorylation System in Cardiac Mitochondria. Int. J. Mol. Sci. 2021, 22, 1462. [Google Scholar] [CrossRef]
- Davies, K.M.; Strauss, M.; Daum, B.; Kief, J.H.; Osiewacz, H.D.; Rycovska, A.; Zickermann, V.; Kühlbrandt, W. Macromolecular Organization of ATP Synthase and Complex I in Whole Mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, 14121–14126. [Google Scholar] [CrossRef]
- Strauss, M.; Hofhaus, G.; Schröder, R.R.; Kühlbrandt, W. Dimer Ribbons of ATP Synthase Shape the Inner Mitochondrial Membrane. EMBO J. 2008, 27, 1154–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikon, N.; Ryan, R.O. Cardiolipin and Mitochondrial Cristae Organization. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Heredia, E.; Tsai, F.-C.; Salinas-Almaguer, S.; Cao, F.J.; Bassereau, P.; Monroy, F. Membrane Curvature Induces Cardiolipin Sorting. Commun. Biol. 2019, 2, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnarez, C.; Marrink, S.J.; Periole, X. Identification of Cardiolipin Binding Sites on Cytochrome c Oxidase at the Entrance of Proton Channels. Sci. Rep. 2013, 3, 1263. [Google Scholar] [CrossRef] [Green Version]
- Arias-Cartin, R.; Grimaldi, S.; Arnoux, P.; Guigliarelli, B.; Magalon, A. Cardiolipin Binding in Bacterial Respiratory Complexes: Structural and Functional Implications. Biochim. Biophys. Acta (BBA)-Bioenerg. 2012, 1817, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, K.; Klingenberg, M. ADP/ATP Carrier Protein from Beef Heart Mitochondria Has High Amounts of Tightly Bound Cardiolipin, as Revealed by Phosphorus-31 Nuclear Magnetic Resonance. Biochemistry 1985, 24, 3821–3826. [Google Scholar] [CrossRef]
- Gasanov, S.E.; Kim, A.A.; Yaguzhinsky, L.S.; Dagda, R.K. Non-Bilayer Structures in Mitochondrial Membranes Regulate ATP Synthase Activity. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 586–599. [Google Scholar] [CrossRef]
- Mühleip, A.; McComas, S.E.; Amunts, A. Structure of a Mitochondrial ATP Synthase with Bound Native Cardiolipin. eLife 2019, 8, e51179. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Ruggiero, F.M. Reactive Oxygen Species Affect Mitochondrial Electron Transport Complex I Activity through Oxidative Cardiolipin Damage. Gene 2002, 286, 135–141. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Ruggiero, F.M. Reactive Oxygen Species Generated by the Mitochondrial Respiratory Chain Affect the Complex III Activity via Cardiolipin Peroxidation in Beef-Heart Submitochondrial Particles. Mitochondrion 2001, 1, 151–159. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Ruggiero, F.M. The Effect of Reactive Oxygen Species Generated from the Mitochondrial Electron Transport Chain on the Cytochrome c Oxidase Activity and on the Cardiolipin Content in Bovine Heart Submitochondrial Particles. FEBS Lett. 2000, 466, 323–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garab, G.; Yaguzhinsky, L.S.; Dlouhý, O.; Nesterov, S.V.; Špunda, V.; Gasanoff, E.S. Structural and Functional Roles of Non-Bilayer Lipid Phases of Chloroplast Thylakoid Membranes and Mitochondrial Inner Membranes. Prog. Lipid Res. 2022, 86, 101163. [Google Scholar] [CrossRef] [PubMed]
- Kunji, E.R.S.; Ruprecht, J.J. The Mitochondrial ADP/ATP Carrier Exists and Functions as a Monomer. Biochem. Soc. Trans. 2020, 48, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, K.; Gohil, V.; Stuart, R.A.; Hunte, C.; Brandt, U.; Greenberg, M.L.; Schägger, H. Cardiolipin Stabilizes Respiratory Chain Supercomplexes. J. Biol. Chem. 2003, 278, 52873–52880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Gluing the Respiratory Chain Together Cardiolipin Is Required for Supercomplex Formation in the Inner Mitochondrial Membrane. J. Biol. Chem. 2002, 277, 43553–43556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Mileykovskaya, E.; Dowhan, W. Cardiolipin Is Essential for Organization of Complexes III and IV into a Supercomplex in Intact Yeast Mitochondria. J. Biol. Chem. 2005, 280, 29403–29408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joubert, F.; Puff, N. Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems. Membranes 2021, 11, 465. [Google Scholar] [CrossRef]
- Kooijman, E.E.; Swim, L.A.; Graber, Z.T.; Tyurina, Y.Y.; Bayır, H.; Kagan, V.E. Magic Angle Spinning 31P NMR Spectroscopy Reveals Two Essentially Identical Ionization States for the Cardiolipin Phosphates in Phospholipid Liposomes. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 61–68. [Google Scholar] [CrossRef]
- Duncan, A.L.; Robinson, A.J.; Walker, J.E. Cardiolipin Binds Selectively but Transiently to Conserved Lysine Residues in the Rotor of Metazoan ATP Synthases. Proc. Natl. Acad. Sci. USA 2016, 113, 8687–8692. [Google Scholar] [CrossRef] [Green Version]
- Mileykovskaya, E.; Dowhan, W. Cardiolipin Membrane Domains in Prokaryotes and Eukaryotes. Biochim. Biophys. Acta 2009, 1788, 2084–2091. [Google Scholar] [CrossRef]
- Huang, K.C.; Mukhopadhyay, R.; Wingreen, N.S. A Curvature-Mediated Mechanism for Localization of Lipids to Bacterial Poles. PLoS Comput. Biol. 2006, 2, e151. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Endo, K.; Wada, H. Specific Distribution of Phosphatidylglycerol to Photosystem Complexes in the Thylakoid Membrane. Front. Plant Sci. 2017, 8, 1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heden, T.D.; Johnson, J.M.; Ferrara, P.J.; Eshima, H.; Verkerke, A.R.P.; Wentzler, E.J.; Siripoksup, P.; Narowski, T.M.; Coleman, C.B.; Lin, C.-T.; et al. Mitochondrial PE Potentiates Respiratory Enzymes to Amplify Skeletal Muscle Aerobic Capacity. Sci. Adv. 2019, 5, eaax8352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikita, H.; Saito, K. Proton Transfer Reactions and Hydrogen-Bond Networks in Protein Environments. J. R. Soc. Interface 2014, 11, 20130518. [Google Scholar] [CrossRef]
- Duan, X.; Scheiner, S.; Wang, R. Modeling Proton Transfer Potentials in Angularly Deformed Hydrogen Bonds. Int. J. Quantum Chem. 1993, 48, 77–87. [Google Scholar] [CrossRef]
- Lemmin, T.; Bovigny, C.; Lançon, D.; Dal Peraro, M. Cardiolipin Models for Molecular Simulations of Bacterial and Mitochondrial Membranes. J. Chem. Theory Comput. 2013, 9, 670–678. [Google Scholar] [CrossRef]
- Griesbauer, J.; Wixforth, A.; Schneider, M.F. Wave Propagation in Lipid Monolayers. Biophys. J. 2009, 97, 2710–2716. [Google Scholar] [CrossRef] [Green Version]
- Heimburg, T.; Jackson, A.D. On Soliton Propagation in Biomembranes and Nerves. Proc. Natl. Acad. Sci. USA 2005, 102, 9790–9795. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, S.; Schneider, M.F. Evidence for Two-Dimensional Solitary Sound Waves in a Lipid Controlled Interface and Its Implications for Biological Signalling. J. R. Soc. Interface 2014, 11, 20140098. [Google Scholar] [CrossRef] [Green Version]
- Prats, M.; Tocanne, J.F.; Teissie, J. Lateral Proton Conduction at a Lipid/Water Interface. Effect of Lipid Nature and Ionic Content of the Aqueous Phase. Eur. J. Biochem. 1987, 162, 379–385. [Google Scholar] [CrossRef]
- Nesterov, S.V.; Skorobogatova, I.A.; Iaguzhinskiĭ, L.S. On specific properties of the mitochondrial oxidative phosphorylation system operating as a supercomplex. Biofizika 2014, 59, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Yaguzhinsky, L.S.; Skorobogatova, Y.A.; Nesterov, S.V. Functionally Significant Low-Temperature Structural Alterations in Mitochondrial Membranes of Homoiothermic Animals. Biophysics 2017, 62, 415–420. [Google Scholar] [CrossRef]
- Fichtl, B.; Shrivastava, S.; Schneider, M.F. Protons at the Speed of Sound: Predicting Specific Biological Signaling from Physics. Sci. Rep. 2016, 6, 22874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haines, T.H.; Dencher, N.A. Cardiolipin: A Proton Trap for Oxidative Phosphorylation. FEBS Lett. 2002, 528, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlberg, M.; Marini, A.; Mennucci, B.; Maliniak, A. Quantum Chemical Modeling of the Cardiolipin Headgroup. J. Phys. Chem. A 2010, 114, 4375–4387. [Google Scholar] [CrossRef]
- Pennington, E.R.; Fix, A.; Sullivan, E.M.; Brown, D.A.; Kennedy, A.; Shaikh, S.R. Distinct Membrane Properties Are Differentially Influenced by Cardiolipin Content and Acyl Chain Composition in Biomimetic Membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 257–267. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ramanathan, A.; Lopez, C.F. Cardiolipin-Dependent Properties of Model Mitochondrial Membranes from Molecular Simulations. Biophys. J. 2019, 117, 429–444. [Google Scholar] [CrossRef] [Green Version]
- Tokutomi, S.; Ohki, K.; Ohnishi, S. Proton-Induced Phase Separation in Phosphatidylserine/Phosphatidylcholine Membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1980, 596, 192–200. [Google Scholar] [CrossRef]
- Angelova, M.I.; Bitbol, A.-F.; Seigneuret, M.; Staneva, G.; Kodama, A.; Sakuma, Y.; Kawakatsu, T.; Imai, M.; Puff, N. PH Sensing by Lipids in Membranes: The Fundamentals of PH-Driven Migration, Polarization and Deformations of Lipid Bilayer Assemblies. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2042–2063. [Google Scholar] [CrossRef]
- Ben-Dov, N.; Korenstein, R. Proton-Induced Endocytosis Is Dependent on Cell Membrane Fluidity, Lipid-Phase Order and the Membrane Resting Potential. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 2672–2681. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M.; Pitaevskii, L.P. Electrodynamics of Continuous Media: Volume 8; Elsevier Science: Amsterdam, The Netherlands, 1995; ISBN 978-0-7506-2634-7. [Google Scholar]
- Abidor, I.G.; Ajtian, S.H.; Chernomordic, L.V.; Cherny, V.V.; Chizmadjev, Y.A. Determination of the Inner Membrane Potential Drop by Potentiodynamic Method. Dokl. Acad. Nauk. 1980, 245, 977–981. [Google Scholar]
- Cherny, V.V.; Sokolov, V.S.; Abidor, I.G. 330-Determination of Surface Charge of Bilayer Lipid Membranes. Bioelectrochem. Bioenerg. 1980, 7, 413–420. [Google Scholar] [CrossRef]
- Jiménez-Munguía, I.; Volynsky, P.E.; Batishchev, O.V.; Akimov, S.A.; Korshunova, G.A.; Smirnova, E.A.; Knorre, D.A.; Sokolov, S.S.; Severin, F.F. Effects of Sterols on the Interaction of SDS, Benzalkonium Chloride, and A Novel Compound, Kor105, with Membranes. Biomolecules 2019, 9, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomès, R.; Roux, B. Free Energy Profiles for H+ Conduction along Hydrogen-Bonded Chains of Water Molecules. Biophys. J. 1998, 75, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pnevmatikos, S. Soliton Dynamics of Hydrogen-Bonded Networks: A Mechanism for Proton Conductivity. Phys. Rev. Lett. 1988, 60, 1534–1537. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterov, S.V.; Yaguzhinsky, L.S.; Vasilov, R.G.; Kadantsev, V.N.; Goltsov, A.N. Contribution of the Collective Excitations to the Coupled Proton and Energy Transport along Mitochondrial Cristae Membrane in Oxidative Phosphorylation System. Entropy 2022, 24, 1813. https://doi.org/10.3390/e24121813
Nesterov SV, Yaguzhinsky LS, Vasilov RG, Kadantsev VN, Goltsov AN. Contribution of the Collective Excitations to the Coupled Proton and Energy Transport along Mitochondrial Cristae Membrane in Oxidative Phosphorylation System. Entropy. 2022; 24(12):1813. https://doi.org/10.3390/e24121813
Chicago/Turabian StyleNesterov, Semen V., Lev S. Yaguzhinsky, Raif G. Vasilov, Vasiliy N. Kadantsev, and Alexey N. Goltsov. 2022. "Contribution of the Collective Excitations to the Coupled Proton and Energy Transport along Mitochondrial Cristae Membrane in Oxidative Phosphorylation System" Entropy 24, no. 12: 1813. https://doi.org/10.3390/e24121813