Novel Three-Dimensional and Non-Invasive Diagnostic Approach for Distinction between Odontogenic Keratocysts and Ameloblastomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Selection and Data Collection
2.2. Hounsfield Unit-Based Quantification of Lesions
2.3. Statistical Analysis
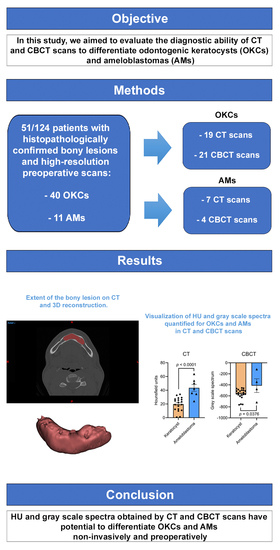
3. Results
3.1. Characterization of the Participant Cohorts
3.2. Hounsfield Unit Spectra Discriminates OKCs from AMs
3.3. HU-Based Discrimination of OKCs and AMs Is Independent from Participant Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. World Health Organization Classification of Head and Neck Tumours, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Wright, J.M.; Vered, M. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and Maxillofacial Bone Tumors. Head Neck Pathol. 2017, 11, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Johnson, N.R.; Gannon, O.M.; Savage, N.W.; Batstone, M.D. Frequency of odontogenic cysts and tumors: A systematic review. J. Investig. Clin. Dent. 2014, 5, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tamiolakis, P.; Thermos, G.; Tosios, K.I.; Sklavounou-Andrikopoulou, A. Demographic and Clinical Characteristics of 5294 Jaw Cysts: A Retrospective Study of 38 Years. Head Neck Pathol. 2019, 13, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Reichart, P.A.; Philipsen, H.P.; Sonner, S. Ameloblastoma: Biological profile of 3677 cases. Eur. J. Cancer Part B Oral Oncol. 1995, 31, 86–99. [Google Scholar] [CrossRef]
- Gohel, A.; Oda, M.; Katkar, A.S.; Sakai, O. Multidetector Row Computed Tomography in Maxillofacial Imaging. Dent. Clin. N. Am. 2018, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Kitisubkanchana, J.; Reduwan, N.H.; Poomsawat, S.; Pornprasertsuk-Damrongsri, S.; Wongchuensoontorn, C. Odontogenic keratocyst and ameloblastoma: Radiographic evaluation. Oral Radiol. 2021, 37, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Rajendra Santosh, A.B. Odontogenic Cysts. Dent. Clin. N. Am. 2020, 64, 105–119. [Google Scholar] [CrossRef]
- Rajendra Santosh, A.B.; Ogle, O.E. Odontogenic Tumors. Dent. Clin. N. Am. 2020, 64, 121–138. [Google Scholar] [CrossRef]
- Bilodeau, E.A.; Seethala, R.R. Update on Odontogenic Tumors: Proceedings of the North American Head and Neck Pathology Society. Head Neck Pathol. 2019, 13, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Murakami, S.; Kishino, M.; Matsuya, T.; Sakuda, M.; Fuchihata, H. Diagnostic imaging in two cases of recurrent maxillary ameloblastoma: Comparative evaluation of plain radiographs, CT and MR images. Br. J. Oral Maxillofac. Surg. 1998, 36, 304–310. [Google Scholar] [CrossRef]
- Bispo, M.S.; Pierre Júnior, M.; Apolinário, A.L., Jr.; Dos Santos, J.N.; Junior, B.C.; Neves, F.S.; Crusoé-Rebello, I. Computer tomographic differential diagnosis of ameloblastoma and odontogenic keratocyst: Classification using a convolutional neural network. Dentomaxillofac. Radiol. 2021, 50, 20210002. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Montes, C.; Mosqueda-Taylor, A.; Carlos-Bregni, R.; de León, E.R.; Palma-Guzmán, J.M.; Páez-Valencia, C.; Meneses-García, A. Ameloblastomas: A regional Latin-American multicentric study. Oral Dis. 2007, 13, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.W.N. Cysts. In White and Pharoah’s Oral Radiology: Principles and Interpretation, 8th ed.; Mallya, S., Lam, E., Eds.; Elsevier: St. Louis, MO, USA, 2019. [Google Scholar]
- Hendra, F.N.; Natsir Kalla, D.S.; Van Cann, E.M.; de Vet, H.C.W.; Helder, M.N.; Forouzanfar, T. Radical vs conservative treatment of intraosseous ameloblastoma: Systematic review and meta-analysis. Oral Dis. 2019, 25, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fan, X.; Su, L.; Wang, Z. Diffusion-Weighted MR Imaging of Unicystic Odontogenic Tumors for Differentiation of Unicystic Ameloblastomas from Keratocystic Odontogenic Tumors. Korean J. Radiol. 2018, 19, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Tozaki, M.; Sugisaki, M.; Yoshida, N.; Fukuda, K.; Tanabe, H. Dynamic multislice helical CT of ameloblastoma and odontogenic keratocyst: Correlation between contrast enhancement and angiogenesis. J. Comput. Assist. Tomogr. 2002, 26, 922–926. [Google Scholar] [CrossRef]
- Kakimoto, N.; Chindasombatjaroen, J.; Tomita, S.; Shimamoto, H.; Uchiyama, Y.; Hasegawa, Y.; Kishino, M.; Murakami, S.; Furukawa, S. Contrast-enhanced multidetector computerized tomography for odontogenic cysts and cystic-appearing tumors of the jaws: Is it useful? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 104–113. [Google Scholar] [CrossRef]
- Minami, M.; Kaneda, T.; Ozawa, K.; Yamamoto, H.; Itai, Y.; Ozawa, M.; Yoshikawa, K.; Sasaki, Y. Cystic lesions of the maxillomandibular region: MR imaging distinction of odontogenic keratocysts and ameloblastomas from other cysts. Am. J. Roentgenol. 1996, 166, 943–949. [Google Scholar] [CrossRef]
- Sumi, M.; Ichikawa, Y.; Katayama, I.; Tashiro, S.; Nakamura, T. Diffusion-weighted MR imaging of ameloblastomas and keratocystic odontogenic tumors: Differentiation by apparent diffusion coefficients of cystic lesions. Am. J. Neuroradiol. 2008, 29, 1897–1901. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, J.; Zhou, Z.; Zhang, Q.; Wu, H.; Zhai, G.; Han, J. Differential diagnosis of ameloblastoma and odontogenic keratocyst by machine learning of panoramic radiographs. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 415–422. [Google Scholar] [CrossRef]
- Alves, D.B.M.; Tuji, F.M.; Alves, F.A.; Rocha, A.C.; Santos-Silva, A.R.D.; Vargas, P.A.; Lopes, M.A. Evaluation of mandibular odontogenic keratocyst and ameloblastoma by panoramic radiograph and computed tomography. Dentomaxillofac. Radiol. 2018, 47, 20170288. [Google Scholar] [CrossRef]
- Ariji, Y.; Morita, M.; Katsumata, A.; Sugita, Y.; Naitoh, M.; Goto, M.; Izumi, M.; Kise, Y.; Shimozato, K.; Kurita, K.; et al. Imaging features contributing to the diagnosis of ameloblastomas and keratocystic odontogenic tumours: Logistic regression analysis. Dentomaxillofac. Radiol. 2011, 40, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamrahian, A.H.; Ioachimescu, A.G.; Remer, E.M.; Motta-Ramirez, G.; Bogabathina, H.; Levin, H.S.; Reddy, S.; Gill, I.S.; Siperstein, A.; Bravo, E.L. Clinical utility of noncontrast computed tomography attenuation value (hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J. Clin. Endocrinol. Metab. 2005, 90, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OH, W. Whole Body Computed Tomography, 2nd ed.; Blackwell: Cambridge, UK, 1993. [Google Scholar]
- Crusoé-Rebello, I.; Oliveira, C.; Campos, P.S.; Azevedo, R.A.; dos Santos, J.N. Assessment of computerized tomography density patterns of ameloblastomas and keratocystic odontogenic tumors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 604–608. [Google Scholar] [CrossRef]
- Reeves, T.E.; Mah, P.; McDavid, W.D. Deriving Hounsfield units using grey levels in cone beam CT: A clinical application. Dentomaxillofac. Radiol. 2012, 41, 500–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, T.; Tateoka, K.; Saito, Y.; Nakazawa, T.; Yano, M.; Nakata, K.; Someya, M.; Hori, M.; Sakata, K. Method for Converting Cone-Beam CT Values into Hounsfield Units for Radiation Treatment Planning. Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2017, 6, 361–375. [Google Scholar] [CrossRef] [Green Version]
- Razi, T.; Niknami, M.; Alavi Ghazani, F. Relationship between Hounsfield Unit in CT Scan and Gray Scale in CBCT. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 107–110. [Google Scholar] [CrossRef]
- Razi, T.; Emamverdizadeh, P.; Nilavar, N.; Razi, S. Comparison of the Hounsfield unit in CT scan with the gray level in cone-beam CT. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 177–182. [Google Scholar] [CrossRef] [Green Version]
- van Rensburg, L.J.; Nortje, C.J.; Thompson, I. Correlating imaging and histopathology of an odontogenic keratocyst in the nevoid basal cell carcinoma syndrome. Dentomaxillofac. Radiol. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Weissman, J.L.; Snyderman, C.H.; Yousem, S.A.; Curtin, H.D. Ameloblastoma of the maxilla: CT and MR appearance. Am. J. Neuroradiol. 1993, 14, 223–226. [Google Scholar]
- Yoshiura, K.; Higuchi, Y.; Ariji, Y.; Shinohara, M.; Yuasa, K.; Nakayama, E.; Ban, S.; Kanda, S. Increased attenuation in odontogenic keratocysts with computed tomography: A new finding. Dentomaxillofac. Radiol. 1994, 23, 138–142. [Google Scholar] [CrossRef]
- Hertzanu, Y.; Mendelsohn, D.B.; Cohen, M. Computed tomography of mandibular ameloblastoma. J. Comput. Assist. Tomogr. 1984, 8, 220–223. [Google Scholar]
- MacDonald, D. Cone-beam computed tomography and the dentist. J. Investig. Clin. Dent. 2015, 8, e12178. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Yoshiura, K. The missing link in image quality assessment in digital dental radiography. Oral Radiol. 2020, 36, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Uehara, K.; Hisatomi, M.; Munhoz, L.; Kawazu, T.; Yanagi, Y.; Okada, S.; Takeshita, Y.; Saito, E.A.; Asaumi, J. Assessment of Hounsfield unit in the differential diagnosis of odontogenic cysts. Dentomaxillofac. Radiol. 2021, 50, 20200188. [Google Scholar] [CrossRef]
- Titinchi, F.; Nortje, C.J. Keratocystic odontogenic tumor: A recurrence analysis of clinical and radiographic parameters. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Siar, C.H.; Lau, S.H.; Ng, K.H. Ameloblastoma of the jaws: A retrospective analysis of 340 cases in a Malaysian population. J. Oral Maxillofac. Surg. 2012, 70, 608–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonetsu, K.; Bianchi, J.G.; Troulis, M.J.; Curtin, H.D. Unusual CT appearance in an odontogenic keratocyst of the mandible: Case report. Am. J. Neuroradiol. 2001, 22, 1887–1889. [Google Scholar]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography). 1. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef]
- Ikeda, K.; Awai, K.; Mori, T.; Kawanaka, K.; Yamashita, Y.; Nomori, H. Differential diagnosis of ground-glass opacity nodules: CT number analysis by three-dimensional computerized quantification. Chest 2007, 132, 984–990. [Google Scholar] [CrossRef]
- Cheon, M.; Yoo, J.; Kang, K.B. 18F-FDG PET/CT and Whole-Body Bone Scan Findings in Gorlin-Goltz Syndrome. Diagnostics 2023, 13, 2239. [Google Scholar] [CrossRef]
- Yalçin, B.K.; Berberoğlu, H.K.; Aralaşmak, A.; Köseoğlu, B.G.; Çakarer, S.; Tekkesin, M.S.; Çarpar, E.; Kula, O. Evaluation of CT and MRI Imaging Results of Radicular Cysts, Odontogenic Keratocysts, and Dentigerous Cysts and their Contribution to the Differential Diagnosis. Curr. Med. Imaging 2022, 18, 1447–1452. [Google Scholar] [CrossRef]
- Borghesi, A.; Nardi, C.; Giannitto, C.; Tironi, A.; Maroldi, R.; Di Bartolomeo, F.; Preda, L. Odontogenic keratocyst: Imaging features of a benign lesion with an aggressive behaviour. Insights Imaging 2018, 9, 883–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, J.P.P.; Ogawa, C.M.; Silveira, R.V.; Castellano, G.; De Rosa, C.S.; Yasuda, C.L.; Rocha, A.C.; Hasseus, B.; Orhan, K.; Braz-Silva, P.H.; et al. Magnetic resonance imaging texture analysis to differentiate ameloblastoma from odontogenic keratocyst. Sci. Rep. 2022, 12, 20047. [Google Scholar] [CrossRef] [PubMed]
- Konouchi, H.; Asaumi, J.; Yanagi, Y.; Hisatomi, M.; Kawai, N.; Matsuzaki, H.; Kishi, K. Usefulness of contrast enhanced-MRI in the diagnosis of unicystic ameloblastoma. Oral Oncol. 2006, 42, 481–486. [Google Scholar] [CrossRef] [PubMed]




| Category | OKC (n = 40) | AM (n = 11) | Total (n = 51) |
|---|---|---|---|
| Gender: | |||
| Female | 17 (42.50%) | 2 (18.18%) | 20 (39.22%) |
| Male | 23 (57.50%) | 9 (81.82%) | 31 (60.78%) |
| Age (MV in years) | 38.74 (9 to 89) | 55 (42 to 79) | 42.06 (9 to 89) |
| Measurement Modality: | |||
| CT | 19 (47.50%) | 7 (63.64%) | 26 (50.98%) |
| CBCT | 21 (52.50%) | 4 (36.36%) | 25 (49.02%) |
| Location: | |||
| Mandibular front | 1 (2.50%) | 1 (9.10%) | 2 (3.92%) |
| Mandibular angle | 16 (40.00%) | 4 (36.36%) | 20 (39.22%) |
| Mandibular corpus | 15 (37.50%) | 2 (18.18%) | 17 (33.33%) |
| Ramus | 2 (5.00%) | 0 | 2 (3.92%) |
| Maxilla and maxillary sinus | 6 (15.00%) | 4 (36.36%) | 10 (19.61%) |
| Measured volume (MV in mm3) | 4884 (433 to 19,678) | 15,527 (2870 to 48,601) | 7179 (433 to 48,601) |
| Measured number of pixels | 140,733 (2142 to 895,726) | 233,914 (19,415 to 850,491) | 160,831 (2142 to 895,726) |
| Measuring Modality | ||||
|---|---|---|---|---|
| (n = 26) | CT | (n = 25) | CBCT | |
| Entity | OKC | AM | OKC | AM |
| Mean | 19.79 | 43.52 | −564.76 | −413.76 |
| Median | 22.38 | 42.26 | −546.13 | −410.84 |
| SD | 9.73 | 13.39 | 93.08 | 250.69 |
| Minimum | 0.77 | 24.10 | −762.39 | −716.63 |
| Maximum | 34.04 | 63.65 | −455.96 | −116.71 |
| p-value | <0.0001 | 0.0376 | ||
| Measuring Modality | ||||
|---|---|---|---|---|
| CT | CBCT | |||
| Category | OKC | AM | OKC | AM |
| Age | −0.018 p = 0.9433 | 0.661 p = 0.1063 | −0.124 p = 0.6020 | −0.909 p = 0.2737 |
| Measured volume (mm3) | 0.093 p = 0.7045 | −0.410 p = 0.3613 | −0.102 p = 0.662 | −0.555 p = 0.4455 |
| Measured number of pixels | 0.264 p = 0.2747 | −0.369 p = 0.4159 | −0.057 p = 0.805 | 0.736 p = 0.265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taxis, J.; Platz Batista da Silva, N.; Grau, E.; Spanier, G.; Nieberle, F.; Maurer, M.; Spoerl, S.; Meier, J.K.; Ettl, T.; Reichert, T.E.; et al. Novel Three-Dimensional and Non-Invasive Diagnostic Approach for Distinction between Odontogenic Keratocysts and Ameloblastomas. Dent. J. 2023, 11, 193. https://doi.org/10.3390/dj11080193
Taxis J, Platz Batista da Silva N, Grau E, Spanier G, Nieberle F, Maurer M, Spoerl S, Meier JK, Ettl T, Reichert TE, et al. Novel Three-Dimensional and Non-Invasive Diagnostic Approach for Distinction between Odontogenic Keratocysts and Ameloblastomas. Dentistry Journal. 2023; 11(8):193. https://doi.org/10.3390/dj11080193
Chicago/Turabian StyleTaxis, Juergen, Natascha Platz Batista da Silva, Elisabeth Grau, Gerrit Spanier, Felix Nieberle, Michael Maurer, Steffen Spoerl, Johannes K. Meier, Tobias Ettl, Torsten E. Reichert, and et al. 2023. "Novel Three-Dimensional and Non-Invasive Diagnostic Approach for Distinction between Odontogenic Keratocysts and Ameloblastomas" Dentistry Journal 11, no. 8: 193. https://doi.org/10.3390/dj11080193