The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications
Abstract
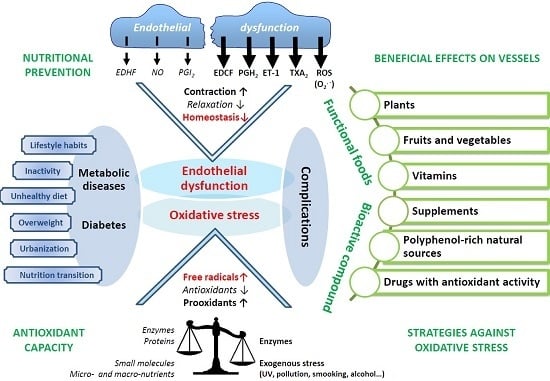
:1. Introduction
2. Diabesity and Cardiovascular Complications
2.1. The Evolution of Obesity and Diabetes
2.2. Lifestyle
2.3. Diabetic Complications: Link with Oxidative Stress and Inflammation
3. Oxidative Stress and Cardiovascular Complications
3.1. Oxidative Stress: A Question of Balance
3.1.1. Oxygen Paradox and Anti-Oxygen
3.1.2. Free Radicals, Oxidative Stress, and Diabetes
3.1.3. Antioxidants Defenses
3.2. Free Radicals: Good and Bad Boys?
3.2.1. Physiological Roles: The Good Boy Side
3.2.2. Pathological Roles: The Bad Boy Side
3.3. Oxidative Stress, Diabetes, and Vascular Complications
3.4. Endothelial Dysfunction, Diabetes, and Complications
3.4.1. Free radicals, NO and NO Synthases
3.4.2. Free Radicals and EDHF
3.4.3. Free Radicals and Contractions
3.4.4. Iron and Non-Transferrin-Bound Iron (NTBI)
4. Nutritional Prevention: Antioxidants against Diabesity and Complications
4.1. Plant Therapy
4.2. Fruits and Vegetables
4.3. Polyphenols: Extract Versus Molecular Compound
4.4. Current Medications
5. Discussion and General Conclusion
Author Contributions
Conflicts of Interest
References
- IDF. IDF Diabetes Atlas, 7th ed.; IDF: Brussels, Belgium, 2015. [Google Scholar]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Orasanu, G.; Plutzky, J. The pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 2009, 53 (Suppl. S5), S35–S42. [Google Scholar] [CrossRef] [PubMed]
- Rosolova, H.; Petrlova, B.; Simon, J.; Sifalda, P.; Sipova, I.; Sefrna, F. Macrovascular and microvascular complications in type 2 diabetes patients. Vnitrni lekarstvi 2008, 54, 229–237. [Google Scholar] [PubMed]
- Sena, C.M.; Pereira, A.M.; Seica, R. Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochimica et Biophysica Acta 2013, 1832, 2216–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Maiese, K.; Targeting, W.N.T. protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol. Histopathol. 2004, 19, 495–504. [Google Scholar] [PubMed]
- You, Z.; Saims, D.; Chen, S.; Zhang, Z.; Guttridge, D.C.; Guan, K.L.; MacDougald, O.A.; Brown, A.M.; Evan, G.; Kitajewski, J.; et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J. Cell Biol. 2002, 157, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Ness, A.R.; Powles, J.W. Fruit and vegetables, and cardiovascular disease: A review. Int. J. Epidemiol. 1997, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van’T Veer, P.; Jansen, M.C.; Klerk, M.; Kok, F.J. Fruits and vegetables in the prevention of cancer and cardiovascular disease. Public Health Nutr. 2000, 3, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Serdula, M.K.; Liu, S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr. Atheroscler. Rep. 2003, 5, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Colditz, G.; Ascherio, A.; Rosner, B.; Spiegelman, D.; et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001, 134, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.J.; Ascherio, A.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Hennekens, C.H.; Spiegelman, D.; Willett, W.C. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 1999, 282, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Dodda, D.; Ciddi, V. Plants used in the management of diabetic complications. Indian J. Pharm. Sci. 2014, 76, 97–106. [Google Scholar] [PubMed]
- Zatalia, S.R.; Sanusi, H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med. Indones. 2013, 45, 141–147. [Google Scholar] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Innes, K.E.; Vincent, K.R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes. Metab. 2007, 9, 813–839. [Google Scholar] [CrossRef] [PubMed]
- Al-Awwadi, N.; Azay, J.; Poucheret, P.; Cassanas, G.; Krosniak, M.; Auger, C.; Gasc, F.; Rouanet, J.M.; Cros, G.; Teissedre, P.L. Antidiabetic activity of red wine polyphenolic extract, ethanol, or both in streptozotocin-treated rats. J. Agric. Food Chem. 2004, 52, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Al-Awwadi, N.A.; Bornet, A.; Azay, J.; Araiz, C.; Delbosc, S.; Cristol, J.P.; Linck, N.; Cros, G.; Teissedre, P.L. Red wine polyphenols alone or in association with ethanol prevent hypertension, cardiac hypertrophy, and production of reactive oxygen species in the insulin-resistant fructose-fed rat. J. Agric. Food Chem. 2004, 52, 5593–5597. [Google Scholar] [CrossRef] [PubMed]
- Schini-Kerth, V.B.; Etienne-Selloum, N.; Chataigneau, T.; Auger, C. Vascular protection by natural product-derived polyphenols: In vitro and in vivo evidence. Planta Med. 2011, 77, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochimica et Biophysica Acta 2012, 1820, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013, 14, 21525–21550. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, M.; Chopra, K. Nordihydroguairetic acid, a lignin, prevents oxidative stress and the development of diabetic nephropathy in rats. Pharmacology 2004, 72, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Koppolu, P.; Chakrabarti, S.; Chen, S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic. Res. 2003, 37, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Choi, Y.H.; Lee, S.J.; Choi, S.A.; Lee, J.H.; Kim, H.; Hong, E.K. Water extract of Aralia elata prevents cataractogenesis in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sytze Van Dam, P.; Cotter, M.A.; Bravenboer, B.; Cameron, N.E. Pathogenesis of diabetic neuropathy: Focus on neurovascular mechanisms. Eur. J. Pharmacol. 2013, 719, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Abdollahi, M. Diabetic neuropathy and oxidative stress: Therapeutic perspectives. Oxid. Med. Cell. Longev. 2013, 2013, 168039. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. A1C variability and the risk of microvascular complications in type 1 diabetes: Data from the Diabetes Control and Complications Trial. Diabetes Care 2008, 31, 2198–2202. [Google Scholar] [CrossRef] [PubMed]
- Marcovecchio, M.L.; Dalton, R.N.; Chiarelli, F.; Dunger, D.B. A1C variability as an independent risk factor for microalbuminuria in young people with type 1 diabetes. Diabetes Care 2011, 34, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Waden, J.; Forsblom, C.; Thorn, L.M.; Gordin, D.; Saraheimo, M.; Groop, P.H. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes 2009, 58, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.; Kawai, K.; Motohashi, S.; Saito, K.; Kodama, S.; Yachi, Y.; Hirasawa, R.; Shimano, H.; Yamazaki, K.; Sone, H. HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia 2012, 55, 2128–2131. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Solini, A.; Bonora, E.; Fondelli, C.; Orsi, E.; Zerbini, G.; Morano, S.; Cavalot, F.; Lamacchia, O.; Laviola, L.; et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: The Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care 2013, 36, 2301–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Group, A.C.; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Molecular and cellular mechanisms in vascular injury in hypertension: Role of angiotensin II. Curr. Opin. Nephrol. Hypertens. 2005, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T. Molecular hydrogen: New antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr. Pharm. Des. 2013, 19, 6375–6381. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Van Linthout, S.; Dhayat, S.; Dhayat, N.; Schmidt, A.; Noutsias, M.; Song, X.-Y.; Spillmann, F.; Riad, A.; Schultheiss, H.-P.; et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 2007, 102, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [PubMed]
- Koppenol, W.H. The Haber-Weiss cycle--70 years later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Fogari, E.; D'Angelo, A.; Bianchi, L.; Bonaventura, A.; Romano, D.; Maffioli, P. Adipocytokine levels in obese and non-obese subjects: An observational study. Inflammation 2013, 36, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wheatley, C.M.; Richards, S.M.; Barrett, E.J.; Clark, M.G.; Rattigan, S. TNF-alpha acutely inhibits vascular effects of physiological but not high insulin or contraction. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E654–E660. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, Y.; Inokuchi, T.; Yamamoto, A.; Ka, T.; Tsutsumi, Z.; Takahashi, S.; Yamamoto, T. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 2007, 44, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Pena-Orihuela, P.; Perez-Martinez, P.; Fuentes, F.; Marin, C.; Tunez, I.; Tinahones, F.J.; Perez-Jimenez, F.; Roche, H.M.; et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp. Mol. Med. 2013, 45, e28. [Google Scholar] [CrossRef] [PubMed]
- Gerschman, R.; Gilbert, D.L.; Nye, S.W.; Dwyer, P.; Fenn, W.O. Oxygen poisoning and x-irradiation: A mechanism in common. Science 1954, 119, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- McCord, J.M.; Fridovich, I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 1969, 244, 6056–6063. [Google Scholar] [PubMed]
- Moureu, C.D.C. Sur l'autoxydation: Essai sur le mécanisme de l'action antioxygène. CR Acad. Sci. (Paris) 1923, 176, 624–629. [Google Scholar]
- Mittal, C.K.; Murad, F. Activation of guanylate cyclase by superoxide dismutase and hydroxyl radical: A physiological regulator of guanosine 3′,5′-monophosphate formation. Proc. Natl. Acad. Sci. USA 1977, 74, 4360–4364. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Nyberg, F. Contribution of environmental factors to cancer risk. Br. Med. Bull. 2003, 68, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.K.; Lalonde, C.; Demling, R. Oxidants and the pathophysiology of burn and smoke inhalation injury. Free Rad. Biol. Med. 1992, 12, 409–415. [Google Scholar] [CrossRef]
- Black, H.S. Potential involvement of free radical reactions in ultraviolet light-mediated cutaneous damage. Photochem. Photobiol. 1987, 46, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, C.; Servy, C.; Cudic, M.; Blanchard, E.B. Intervention by nitric oxide, NO, and its oxide derivatives particularly in mammals. Can. J. Physiol. Pharmacol. 2001, 79, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [PubMed]
- Al Ghouleh, I.; Khoo, N.K.; Knaus, U.G.; Griendling, K.K.; Touyz, R.M.; Thannickal, V.J.; Barchowsky, A.; Nauseef, W.M.; Kelley, E.E.; Bauer, P.M.; et al. Oxidases and peroxidases in cardiovascular and lung disease: New concepts in reactive oxygen species signaling. Free Rad. Biol. Med. 2011, 51, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Selemidis, S.; Sobey, C.G.; Wingler, K.; Schmidt, H.H.; Drummond, G.R. NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol. Ther. 2008, 120, 254–291. [Google Scholar] [CrossRef] [PubMed]
- Dal-Ros, S.; Zoll, J.; Lang, A.L.; Auger, C.; Keller, N.; Bronner, C.; Geny, B.; Schini-Kerth, V.B. Chronic intake of red wine polyphenols by young rats prevents aging-induced endothelial dysfunction and decline in physical performance: Role of NADPH oxidase. Biochem. Biophys. Res. Commun. 2011, 404, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Dal-Ros, S.; Oswald-Mammosser, M.; Pestrikova, T.; Schott, C.; Boehm, N.; Bronner, C.; Chataigneau, T.; Geny, B.; Schini-Kerth, V.B. Losartan prevents portal hypertension-induced, redox-mediated endothelial dysfunction in the mesenteric artery in rats. Gastroenterology 2010, 138, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Dal-Ros, S.; Bronner, C.; Schott, C.; Kane, M.O.; Chataigneau, M.; Schini-Kerth, V.B.; Chataigneau, T. Angiotensin II-induced hypertension is associated with a selective inhibition of endothelium-derived hyperpolarizing factor-mediated responses in the rat mesenteric artery. J. Pharmacol. Exp. Ther. 2009, 328, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Shah, A.M. ROS generation by nonphagocytic NADPH oxidase: Potential relevance in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14 (8 Suppl. S3), S221–S226. [Google Scholar] [CrossRef] [PubMed]
- Dal, S.; Jeandidier, N.; Seyfritz, E.; Bietiger, W.; Peronet, C.; Moreau, F.; Pinget, M.; Maillard, E.; Sigrist, S. Oxidative stress status and liver tissue defenses in diabetic rats during intensive subcutaneous insulin therapy. Exp. Biol. Med. 2016, 241, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Dal, S.; Jeandidier, N.; Schaschkow, A.; Spizzo, A.H.; Seyfritz, E.; Sookhareea, C.; Bietiger, W.; Peronet, C.; Moreau, F.; Pinget, M.; et al. Portal or subcutaneous insulin infusion: Efficacy and impact on liver inflammation. Fundam. Clin. Pharmacol. 2015, 29, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Morgan, D.; Rebelato, E.; Oliveira-Emilio, H.C.; Procopio, J.; Curi, R.; Carpinelli, A. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 2009, 52, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schroder, K. Nox family NADPH oxidases in mechano-transduction: Mechanisms and consequences. Antioxid. Redox Signal. 2014, 20, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.; Morris, A.D.; Belch, J.J.; Hill, A.; Struthers, A.D. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 2000, 35, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [PubMed]
- Bonnefont-Rousselot, D.; Raji, B.; Walrand, S.; Gardes-Albert, M.; Jore, D.; Legrand, A.; Peynet, J.; Vasson, M.P. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism 2003, 52, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.; Halliwell, B. Micronutrients: Oxidant/antioxidant status. Br. J. Nutr. 2001, 85 (Suppl. 2), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Prieto, M.; Biarnes, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, R.; Vreven, T.; Morokuma, K.; Musaev, D.G. Elucidation of the mechanism of selenoprotein glutathione peroxidase (GPx)-catalyzed hydrogen peroxide reduction by two glutathione molecules: A density functional study. Biochemistry 2005, 44, 11864–11871. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Bounous, G. Whey protein concentrate (WPC) and glutathione modulation in cancer treatment. Anticancer Res. 2000, 20, 4785–4792. [Google Scholar] [PubMed]
- Thornalley, P.J.; McLellan, A.C.; Lo, T.W.; Benn, J.; Sonksen, P.H. Negative association between erythrocyte reduced glutathione concentration and diabetic complications. Clin. Sci. 1996, 91, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Grankvist, K.; Marklund, S.L.; Taljedal, I.B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 1981, 199, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Rad. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Acharya, J.D.; Ghaskadbi, S.S. Islets and their antioxidant defense. Islets 2010, 2, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Hagele, R.; Youssif, N.; Eujen, R.; El-Amri, N. A possible role of intracellular and membrane thiols of rat pancreatic islets in calcium uptake and insulin release. Endocrinology 1983, 112, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of reactive oxygen species in biological processes. Klini. Wochenschr. 1991, 69, 965–968. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [PubMed]
- Lowenstein, C.J.; Dinerman, J.L.; Snyder, S.H. Nitric oxide: A physiologic messenger. Ann. Intern. Med. 1994, 120, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W. Redox regulation of transcriptional activators. Free Rad. Biol. Med. 1996, 21, 335–348. [Google Scholar] [CrossRef]
- Frenette, P.S.; Wagner, D.D. Adhesion molecules--Part 1. N. Engl. J. Med. 1996, 334, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K. NADPH oxidases in redox regulation of cell adhesion and migration. Antioxid. Redox Signal. 2014, 20, 2043–2058. [Google Scholar] [CrossRef] [PubMed]
- Keisari, Y.; Braun, L.; Flescher, E. The oxidative burst and related phenomena in mouse macrophages elicited by different sterile inflammatory stimuli. Immunobiology 1983, 165, 78–89. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, K.; Lei, Y.; Li, Q.; Nice, E.C.; Huang, C. Redox signaling: Potential arbitrator of autophagy and apoptosis in therapeutic response. Free Rad. Biol. Med. 2015, 89, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Richard, V.J.; Reimer, K.A.; Jennings, R.B. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ. Res. 1990, 66, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Kalikiri, P.C.; Sachan, R.S.G.S. Ischemic and anesthetic preconditioning of the heart: An insith into concepts and mechanisms. Internet J. Anesthesiol. 2004, 8, 2. [Google Scholar]
- Zhao, T.C.; Hines, D.S.; Kukreja, R.C. Adenosine-induced late preconditioning in mouse hearts: Role of p38 MAP kinase and mitochondrial K(ATP) channels. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1278–H1285. [Google Scholar] [PubMed]
- Laude, K.; Favre, J.; Thuillez, C.; Richard, V. NO produced by endothelial NO synthase is a mediator of delayed preconditioning-induced endothelial protection. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H2053–H2060. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, S.; Arnold, W.; Mittal, C.; Murad, F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J. Cycl. Nucleotide Res. 1977, 3, 23–35. [Google Scholar]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. Endothelium and control of vascular function. State of the Art lecture. Hypertension 1989, 13 6 Pt 2, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. Role of the endothelium in control of vascular smooth muscle function. Verhandelingen—Koninklijke Academie voor Geneeskunde van Belgie 1982, 44, 411–418. [Google Scholar] [PubMed]
- Schini-Kerth, V.B. Vascular biosynthesis of nitric oxide: Effect on hemostasis and fibrinolysis. Transfusion clinique et biologique 1999, 6, 355–363. [Google Scholar] [CrossRef]
- Prosser, B.L.; Ward, C.W.; Lederer, W.J. X-ROS signaling: Rapid mechano-chemo transduction in heart. Science 2011, 333, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P.; Lawrence, J.C., Jr.; Lynn, W.S. Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc. Natl. Acad. Sci. USA 1974, 71, 4173–4177. [Google Scholar] [CrossRef] [PubMed]
- Higaki, Y.; Mikami, T.; Fujii, N.; Hirshman, M.F.; Koyama, K.; Seino, T.; Tanaka, K.; Goodyear, L.J. Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E889–E897. [Google Scholar] [CrossRef] [PubMed]
- Christon, R.; Drouin, O.; Marette, A. Redox modulation of insulin signaling and endothelial function. Antioxid. Redox Signal. 2005, 7, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; de Haen, C. The insulin-like effect of hydrogen peroxide on pathways of lipid synthesis in rat adipocytes. J. Biol. Chem. 1979, 254, 9017–9021. [Google Scholar] [PubMed]
- Tiganis, T. Reactive oxygen species and insulin resistance: The good, the bad and the ugly. Trends Pharmacol. Sci. 2011, 32, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M.; et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Bellon, S.; Berger, M.; Bourdat, A.G.; Douki, T.; Duarte, V.; Frelon, S.; Gasparutto, D.; Muller, E.; Ravanat, J.L.; et al. Recent aspects of oxidative DNA damage: Guanine lesions, measurement and substrate specificity of DNA repair glycosylases. Biol. Chem. 2002, 383, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Hehner, S.P.; Breitkreutz, R.; Shubinsky, G.; Unsoeld, H.; Schulze-Osthoff, K.; Schmitz, M.L.; Droge, W. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J. Immunol. 2000, 165, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, N. 4-hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003, 24, 281–291. [Google Scholar] [CrossRef]
- Petersen, D.R.; Doorn, J.A. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Rad. Biol. Med. 2004, 37, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Esterbauer, H.; Gebicki, J.; Puhl, H.; Jurgens, G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Rad. Biol. Med. 1992, 13, 341–390. [Google Scholar] [CrossRef]
- Fraley, A.E.; Tsimikas, S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr. Opin. Lipidol. 2006, 17, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Torzewski, M.; Klouche, M.; Hock, J.; Messner, M.; Dorweiler, B.; Torzewski, J.; Gabbert, H.E.; Bhakdi, S. Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Makita, Z.; Yanagisawa, K.; Kuwajima, S.; Bucala, R.; Vlassara, H.; Koike, T. The role of advanced glycosylation end-products in the pathogenesis of atherosclerosis. Nephrol. Dial. Transplant. 1996, 11 (Suppl. S5), 31–33. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Vannucci, S.J.; Yan, S.S.; Herold, K.; Yan, S.F.; Schmidt, A.M. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 2005, 15, 16R–28R. [Google Scholar] [CrossRef] [PubMed]
- Grimsrud, P.A.; Picklo, M.J., Sr.; Griffin, T.J.; Bernlohr, D.A. Carbonylation of adipose proteins in obesity and insulin resistance: Identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol. Cell. Proteom. MCP 2007, 6, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.E.; Sturgess, A.D.; Davies, M.J. Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2005, 52, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Boldogh, I.; Bacsi, A.; Choudhury, B.K.; Dharajiya, N.; Alam, R.; Hazra, T.K.; Mitra, S.; Goldblum, R.M.; Sur, S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Investig. 2005, 115, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.; Guerrero-Plata, A.; Suarez-Real, G.; Adegboyega, P.A.; Colasurdo, G.N.; Khan, A.M.; Garofalo, R.P.; Casola, A. Antioxidant treatment ameliorates respiratory syncytial virus-induced disease and lung inflammation. Am. J. Respir. Crit. Care Med. 2006, 174, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free Rad. Biol. Med. 2013, 61, 473–501. [Google Scholar] [CrossRef] [PubMed]
- Bloch-Damti, A.; Bashan, N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid. Redox Signal. 2005, 7, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.R.; Thomason, H.; Sandler, D.; Leguen, C.; Baxter, M.A.; Thorpe, G.H.; Jones, A.F.; Barnett, A.H. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur. J. Clin. Investig. 1997, 27, 484–490. [Google Scholar] [CrossRef]
- Rocic, B.; Vucic, M.; Knezevic-Cuca, J.; Radica, A.; Pavlic-Renar, I.; Profozic, V.; Metelko, Z. Total plasma antioxidants in first-degree relatives of patients with insulin-dependent diabetes. Exp. Clin. Endocrinol. Diabetes 1997, 105, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.A.; Marra, G.; Giardina, B.; Cotroneo, P.; Mordente, A.; Martorana, G.E.; Manto, A.; Ghirlanda, G. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes 1997, 46, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853.
- Gaede, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Luscher, T.F.; Creager, M.A.; Beckman, J.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Circulation 2003, 108, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Luscher, T.F.; Cosentino, F.; Beckman, J.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 2003, 108, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA. 2000, 97, 12222–12226. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 2010, 59, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Glucose and reactive oxygen species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Spitaler, M.M.; Graier, W.F. Vascular targets of redox signalling in diabetes mellitus. Diabetologia 2002, 45, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Romero, C.; Sadidi, M.; Feldman, E.L. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev. Endocr. Metab. Disord. 2008, 9, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Defraigne, J.O. A central pathological mechanism explaining diabetic complications? Revue medicale de Liege 2005, 60, 472–478. [Google Scholar] [PubMed]
- Suarez, G.; Rajaram, R.; Bhuyan, K.C.; Oronsky, A.L.; Goidl, J.A. Administration of an aldose reductase inhibitor induces a decrease of collagen fluorescence in diabetic rats. J. Clin. Investig. 1988, 82, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Zheng, F.; Striker, G.E.; Vlassara, H. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am. J. Pathol. 2008, 173, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Wallenstein, S.; Striker, G.E.; et al. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: Association with increased AGER1 expression. Am. J. Pathol. 2007, 170, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, C.; Zhang, X.H.; Zheng, F.; Cai, W.; Vlassara, H.; Ma, Z.A. Advanced glycation end products inhibit glucose-stimulated insulin secretion through nitric oxide-dependent inhibition of cytochrome c oxidase and adenosine triphosphate synthesis. Endocrinology 2009, 150, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Beyan, H.; Riese, H.; Hawa, M.I.; Beretta, G.; Davidson, H.W.; Hutton, J.C.; Burger, H.; Schlosser, M.; Snieder, H.; Boehm, B.O.; et al. Glycotoxin and autoantibodies are additive environmentally determined predictors of type 1 diabetes: A twin and population study. Diabetes 2012, 61, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating glycotoxins and dietary advanced glycation endproducts: Two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.; Ho, E.C.; Lam, K.S.; Chung, S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003, 14 (Suppl. S3), S233–S236. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; Monge, J.C.; Gordon, A.; Cernacek, P.; Blais, D.; Stewart, D.J. Lack of role for nitric oxide (NO) in the selective destabilization of endothelial NO synthase mRNA by tumor necrosis factor-alpha. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 52–57. [Google Scholar] [CrossRef] [PubMed]
- De Keulenaer, G.W.; Alexander, R.W.; Ushio-Fukai, M.; Ishizaka, N.; Griendling, K.K. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem. J. 1998, 329 Pt 3, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Pfaffe, T.; Cooper-White, J.; Beyerlein, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future applications. Clin. Chem. 2011, 57, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241–248. [Google Scholar] [PubMed]
- Su, H.; Velly, A.M.; Salah, M.H.; Benarroch, M.; Trifiro, M.; Schipper, H.M.; Gornitsky, M. Altered redox homeostasis in human diabetes saliva. J. Oral Pathol. Med. 2012, 41, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Gumus, P.; Buduneli, N.; Cetinkalp, S.; Hawkins, S.I.; Renaud, D.; Kinane, D.F.; Scott, D.A. Salivary antioxidants in patients with type 1 or 2 diabetes and inflammatory periodontal disease: A case-control study. J. Periodontol. 2009, 80, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Shehadeh, N.; Shafir, Y.; Nagler, R.M. Free radicals related effects and antioxidants in saliva and serum of adolescents with Type 1 diabetes mellitus. Arch. Oral Biol. 2006, 51, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Astaneie, F.; Afshari, M.; Mojtahedi, A.; Mostafalou, S.; Zamani, M.J.; Larijani, B.; Abdollahi, M. Total antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patients. Arch. Med. Res. 2005, 36, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, G.; Thomas, B.; Joshi, S.R. Evaluation of Total Antioxidant Capacity of Saliva in Type 2 Diabetic Patients with and without Periodontal Disease: A Case-Control Study. N. Am. J. Med. Sci. 2013, 5, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Schipper, H.M.; Velly, A.M.; Mohit, S.; Gornitsky, M. Salivary biomarkers of oxidative stress: A critical review. Free Rad. Biol. Med. 2015, 85, 95–104. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995, 310, 83–88. [Google Scholar]
- De Caterina, R. Endothelial dysfunctions: Common denominators in vascular disease. Curr. Opin. Lipidol. 2000, 11, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Stehouwer, C.D.; Lambert, J.; Donker, A.J.; van Hinsbergh, V.W. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc. Res. 1997, 34, 55–68. [Google Scholar] [CrossRef]
- Desouza, C.V.; Bolli, G.B.; Fonseca, V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care 2010, 33, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Paneni, F.; Cosentino, F.; Creager, M.A. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II. Eur. Heart J. 2013, 34, 2444–2452. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Schmieder, R.E. Impaired endothelial function in arterial hypertension and hypercholesterolemia: Potential mechanisms and differences. J. Hypertens. 2000, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, S. Smoking as a risk factor for endothelial dysfunction. Can. J. Cardiol. 1998, 14, 20D–22D. [Google Scholar] [PubMed]
- Arcaro, G.; Zamboni, M.; Rossi, L.; Turcato, E.; Covi, G.; Armellini, F.; Bosello, O.; Lechi, A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Yasue, H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J. Am. Coll. Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef]
- Vehkavaara, S.; Seppala-Lindroos, A.; Westerbacka, J.; Groop, P.H. Yki-Jarvinen H.in vivo endothelial dysfunction characterizes patients with impaired fasting glucose. Diabetes Care 1999, 22, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. Is insulin resistance atherogenic? Possible mechanisms. Atheroscler. Suppl. 2006, 7, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, E.; DeFronzo, R.A. Insulin resistance and endothelial dysfunction: The road map to cardiovascular diseases. Diabetes/Metab. Res. Rev. 2006, 22, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.P.; Rawal, K.; Bagchi, A.K.; Akolkar, G.; Bernardes, N.; Dias, D.D.; Gupta, S.; Singal, P.K. Insulin resistance: An additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail. Rev. 2016, 21, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. Hypercholesterolaemia, atherosclerosis and release of endothelium-derived relaxing factor by aggregating platelets. Eur. Heart J. 1991, 12 (Suppl.), E:25–E:32. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Boulanger, C.M. Endothelium-dependent responses in hypertension. Hypertens. Res. 1995, 18, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Cleland, S.J.; Sattar, N.; Petrie, J.R.; Forouhi, N.G.; Elliott, H.L.; Connell, J.M. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin. Sci. 2000, 98, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Kamisah, Y.; Faizah, O.; Jaarin, K. The role of repeatedly heated soybean oil in the development of hypertension in rats: Association with vascular inflammation. Int. J. Exp. Pathol. 2012, 93, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.; Prado, K.; Foppa, M.; Martinelli, N.; Aguiar, C.; Furian, T.; Clausell, N.; Rohde, L.E. Endothelial dysfunction assessed by brachial artery ultrasound in severe sepsis and septic shock. J. Crit. Care 2012, 27, 316 e9–316 e14. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.M.; Ferreira, I.; Kostense, P.J.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Kamp, O.; Bouter, L.M.; Stehouwer, C.D. Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not; The Hoorn Study. Atherosclerosis 2004, 174, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; Paquot, N.; Scheen, A.J. Inflammatory markers and cardiometabolic diseases. Acta Clin. Belg. 2015, 70, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Busse, R.; Fleming, I. Endothelial dysfunction in atherosclerosis. J. Vasc. Res. 1996, 33, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Barton, M. Endothelial dysfunction and atherosclerosis: Endothelin receptor antagonists as novel therapeutics. Curr. Hypertens. Rep. 2000, 2, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bousette, N.; Giaid, A. Endothelin-1 in atherosclerosis and other vasculopathies. Can. J. Physiol. Pharmacol. 2003, 81, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Sorensen, K.E.; Gooch, V.M.; Spiegelhalter, D.J.; Miller, O.I.; Sullivan, I.D.; Lloyd, J.K.; Deanfield, J.E. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 1992, 340, 1111–1115. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Andrawis, N.; Jones, D.S.; Abernethy, D.R. Aging is associated with endothelial dysfunction in the human forearm vasculature. J. Am. Geriatr. Soc. 2000, 48, 193–198. [Google Scholar] [PubMed]
- Fornoni, A.; Raij, L. Metabolic syndrome and endothelial dysfunction. Curr. Hypertens. Rep. 2005, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, A.K.; Jain, A.; Singh, A.; Goswami, B.; Bhatnagar, M.K.; Bhatacharjee, J. The study of markers of endothelial dysfunction in metabolic syndrome. Horm. Mol. Biol. Clin. Investig. 2015, 24, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; Cuevas, P.; Fernandez, A.; Gabancho, S.; Allona, A.; Martin-Morales, A.; Moncada, I.; Videla, S.; de Tejada, I.S. Diabetes impairs endothelium-dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem. Biophys. Res. Commun. 2003, 312, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Von Scholten, B.J.; Reinhard, H.; Hansen, T.W.; Schalkwijk, C.G.; Stehouwer, C.; Parving, H.H.; Jacobsen, P.K.; Rossing, P. Markers of inflammation and endothelial dysfunction are associated with incident cardiovascular disease, all-cause mortality, and progression of coronary calcification in type 2 diabetic patients with microalbuminuria. J. Diabetes Complicat. 2016, 30, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Pieper, G.M. Review of alterations in endothelial nitric oxide production in diabetes: Protective role of arginine on endothelial dysfunction. Hypertension 1998, 31, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Verbeuren, T.J.; Van de Voorde, J.; Lameire, N.H.; Vanhoutte, P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000, 130, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Tanabe, K.; Croker, B.P.; Johnson, R.J.; Grant, M.B.; Kosugi, T.; Li, Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Ripandelli, G.; Taurone, S.; Feher, J.; Plateroti, R.; Kovacs, I.; Magliulo, G.; Orlando, M.P.; Micera, A.; Battaglione, E.; et al. Age and diabetes related changes of the retinal capillaries: An ultrastructural and immunohistochemical study. Int. J. Immunopathol. Pharmacol. 2016, 29, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; Cuevas, P.; Gabancho, S.; Gonzalez-Corrochano, R.; Videla, S.; Saenz de Tejada, I. Enhancement of both EDHF and NO/cGMP pathways is necessary to reverse erectile dysfunction in diabetic rats. J. Sex. Med. 2005, 2, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Ding, H.; Anderson, T.J.; Pannirselvam, M. The endothelium in health and disease: A discussion of the contribution of non-nitric oxide endothelium-derived vasoactive mediators to vascular homeostasis in normal vessels and in type II diabetes. Mol. Cell. Biochem. 2004, 263, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; FitzGerald, G.A. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circulation 2003, 108, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Kurz, S.; Munzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.J., Jr.; Gutterman, D.D.; Rios, C.D.; Heistad, D.D.; Davidson, B.L. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ. Res. 1998, 82, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Rubanyi, G.M.; Vanhoutte, P.M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986, 250 5 Pt 2, H822–H827. [Google Scholar] [PubMed]
- Price, D.T.; Vita, J.A.; Keaney, J.F., Jr. Redox control of vascular nitric oxide bioavailability. Antioxid. Redox Signal. 2000, 2, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Daiber, A.; Ullrich, V.; Mulsch, A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.H.; Cohen, R.; Ullrich, V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 2004, 11, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Quagliaro, L.; D’Amico, M.; Di Filippo, C.; Marfella, R.; Nappo, F.; Berrino, L.; Rossi, F.; Giugliano, D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes 2002, 51, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.H.; Vanhoutte, P.M. Endothelial dysfunction: A strategic target in the treatment of hypertension? Pflugers Archiv 2010, 459, 995–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhoutte, P.M. Endothelial dysfunction in hypertension. J. hypertens. Suppl. 1996, 14, S83–S93. [Google Scholar] [PubMed]
- Vanhoutte, P.M. Endothelial dysfunction and atherosclerosis. Eur. Heart J. 1997, 18 (Suppl. SE), E19–E29. [Google Scholar] [CrossRef]
- Seppo, L.; Karjala, K.; Nevala, R.; Korpela, R.; Lahteenmaki, T.; Solatunturi, E.; Tikkanen, M.J.; Vapaatalo, H. A long-term fish diet modifies the toxic properties of human partially oxidized LDL on vascular preparations in vitro. J. Physiol. Pharmacol. 2000, 51, 251–265. [Google Scholar] [PubMed]
- Vidal, F.; Colome, C.; Martinez-Gonzalez, J.; Badimon, L. Atherogenic concentrations of native low-density lipoproteins down-regulate nitric-oxide-synthase mRNA and protein levels in endothelial cells. Eur. J. Biochem./FEBS 1998, 252, 378–384. [Google Scholar] [CrossRef]
- Matz, R.L.; de Sotomayor, M.A.; Schott, C.; Stoclet, J.C.; Andriantsitohaina, R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br. J. Pharmacol. 2000, 131, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Matz, R.L.; Schott, C.; Stoclet, J.C.; Andriantsitohaina, R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol. Res./Acad. Scientiarum Bohemoslov. 2000, 49, 11–18. [Google Scholar]
- Dal-Ros, S.; Bronner, C.; Auger, C.; Schini-Kerth, V.B. Red wine polyphenols improve an established aging-related endothelial dysfunction in the mesenteric artery of middle-aged rats: Role of oxidative stress. Biochem. Biophys. Res. Commun. 2012, 419, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Akbari, C.M.; LoGerfo, F.W. Diabetes and peripheral vascular disease. J. Vasc. Surg. 1999, 30, 373–384. [Google Scholar] [CrossRef]
- Wolff, S.P.; Dean, R.T. Glucose autoxidation and protein modification. The potential role of 'autoxidative glycosylation' in diabetes. Biochem. J. 1987, 245, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Ishida, K.; Nakayama, N.; Taguchi, K.; Kobayashi, T.; Kamata, K. Mechanisms underlying the losartan treatment-induced improvement in the endothelial dysfunction seen in mesenteric arteries from type 2 diabetic rats. Pharmacol. Res. 2010, 62, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Morrishow, A.M.; Dharia, N.; Gross, S.S.; Goligorsky, M.S. Glucose scavenging of nitric oxide. Am. J. Physiol. Renal Physiol. 2001, 280, F480–F486. [Google Scholar] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Noiri, E.; Satoh, H.; Taguchi, J.; Brodsky, S.V.; Nakao, A.; Ogawa, Y.; Nishijima, S.; Yokomizo, T.; Tokunaga, K.; Fujita, T. Association of eNOS Glu298Asp polymorphism with end-stage renal disease. Hypertension 2002, 40, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Gao, S.; Li, H.; Goligorsky, M.S. Hyperglycemic switch from mitochondrial nitric oxide to superoxide production in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2130–H2139. [Google Scholar] [CrossRef] [PubMed]
- Komers, R.; Schutzer, W.E.; Reed, J.F.; Lindsley, J.N.; Oyama, T.T.; Buck, D.C.; Mader, S.L.; Anderson, S. Altered endothelial nitric oxide synthase targeting and conformation and caveolin-1 expression in the diabetic kidney. Diabetes 2006, 55, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Tsutsui, M.; Shimokawa, H.; Suda, O.; Morishita, T.; Shibata, K.; Tanimoto, A.; Nagasaki, M.; Tasaki, H.; Sasaguri, Y.N.; et al. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation 2008, 117, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Shimokawa, H.; Yanagihara, N.; Otsuji, Y.; Tsutsui, M. Nitric oxide synthases and heart failure—Lessons from genetically manipulated mice. J. UOEH 2013, 35, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Singh, G.B.; Khullar, M. Nitric oxide synthases and diabetic cardiomyopathy. Nitric Oxide 2014, 43, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Carnicer, R.; Crabtree, M.J.; Sivakumaran, V.; Casadei, B.; Kass, D.A. Nitric oxide synthases in heart failure. Antioxid. Redox Signal. 2013, 18, 1078–1099. [Google Scholar] [CrossRef] [PubMed]
- Komers, R.; Lindsley, J.N.; Oyama, T.T.; Allison, K.M.; Anderson, S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am. J. Physiol. Renal Physiol. 2000, 279, F573–F583. [Google Scholar] [PubMed]
- Watkins, C.C.; Sawa, A.; Jaffrey, S.; Blackshaw, S.; Barrow, R.K.; Snyder, S.H.; Ferris, C.D. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J. Clin. Investig. 2000, 106, 803. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [PubMed]
- Nathan, C.; Xie, Q.W. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Weldy, C.S.; Wilkerson, H.W.; Larson, T.V.; Stewart, J.A.; Kavanagh, T.J. DIESEL particulate exposed macrophages alter endothelial cell expression of eNOS, iNOS, MCP1, and glutathione synthesis genes. Toxicol. in Vitro 2011, 25, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Otani, H.; Jo, F.; Shimazu, T.; Okazaki, T.; Yoshioka, K.; Fujita, M.; Kosaki, A.; Iwasaka, T. Inhibition of nitric oxide synthase uncoupling by sepiapterin improves left ventricular function in streptozotocin-induced diabetic mice. Clin. Exp. Pharmacol. Physiol. 2011, 38, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Nagareddy, P.R.; Soliman, H.; Lin, G.; Rajput, P.S.; Kumar, U.; McNeill, J.H.; MacLeod, K.M. Selective inhibition of protein kinase C beta(2) attenuates inducible nitric oxide synthase-mediated cardiovascular abnormalities in streptozotocin-induced diabetic rats. Diabetes 2009, 58, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Gador, A.; Lu, Y.H.; Lin, G.; Bankar, G.; MacLeod, K.M. Diabetes-induced increased oxidative stress in cardiomyocytes is sustained by a positive feedback loop involving Rho kinase and PKCbeta2. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H989–H1000. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Li, H.; Xu, J.; Liu, Y.; Gao, X.; Wang, J.; Ng, K.F.; Lau, W.B.; Ma, X.L.; Rodrigues, B.; et al. Hyperglycemia-induced protein kinase C beta2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes 2013, 62, 2318–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feletou, M.; Vanhoutte, P.M. EDHF: New therapeutic targets? Pharmacol. Res. 2004, 49, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Mauricio, M.D.; Aldasoro, M.; Ortega, J.; Vila, J.M. Endothelial dysfunction in morbid obesity. Curr. Pharm. Des. 2013, 19, 5718–5729. [Google Scholar] [CrossRef] [PubMed]
- Rvdw, I.L.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; Dal, S. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2015. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Mechanisms underlying the impaired EDHF-type relaxation response in mesenteric arteries from Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Eur. J. Pharmacol. 2006, 538, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Burnham, M.P.; Johnson, I.T.; Weston, A.H. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in Type II diabetic ZDF rats. Br. J. Pharmacol. 2006, 148, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Agouni, A.; Lagrue-Lak-Hal, A.H.; Mostefai, H.A.; Tesse, A.; Mulder, P.; Rouet, P.; Desmoulin, F.; Heymes, C.; Martinez, M.C.; Andriantsitohaina, R. Red wine polyphenols prevent metabolic and cardiovascular alterations associated with obesity in Zucker fatty rats (Fa/Fa). PLoS ONE 2009, 4, e5557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brondum, E.; Kold-Petersen, H.; Simonsen, U.; Aalkjaer, C. NS309 restores EDHF-type relaxation in mesenteric small arteries from type 2 diabetic ZDF rats. Br. J. Pharmacol. 2010, 159, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Noguchi, E.; Ishida, K.; Nakayama, N.; Kobayashi, T.; Kamata, K. Cilostazol improves endothelial dysfunction by increasing endothelium-derived hyperpolarizing factor response in mesenteric arteries from Type 2 diabetic rats. Eur. J. Pharmacol. 2008, 599, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.H.; Vanhoutte, P.M. Prostanoids and reactive oxygen species: Team players in endothelium-dependent contractions. Pharmacol. Ther. 2009, 122, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Tang, E.H. Endothelium-dependent contractions: when a good guy turns bad! J. Physiol. 2008, 586 Pt 22, 5295–5304. [Google Scholar] [CrossRef] [PubMed]
- Basuli, D.; Stevens, R.G.; Torti, F.M.; Torti, S.V. Epidemiological associations between iron and cardiovascular disease and diabetes. Front. Pharmacol. 2014, 5, 117. [Google Scholar] [PubMed]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loreal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochimica et Biophysica Acta 2012, 1820, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Aljwaid, H.; White, D.L.; Collard, K.J.; Moody, A.J.; Pinkney, J.H. Non-transferrin-bound iron is associated with biomarkers of oxidative stress, inflammation and endothelial dysfunction in type 2 diabetes. J. Diabetes Complicat. 2015, 29, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, M.; Asleh, R.; Cabantchik, Z.I.; Breuer, W.; Aronson, D.; Suleiman, A.; Miller-Lotan, R.; Hammerman, H.; Levy, A.P. Serum chelatable redox-active iron is an independent predictor of mortality after myocardial infarction in individuals with diabetes. Diabetes Care 2004, 27, 2730–2732. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Liu, D.Y.; Jacobs, D.R., Jr.; Shin, H.R.; Song, K.; Lee, I.K.; Kim, B.; Hider, R.C. Common presence of non-transferrin-bound iron among patients with type 2 diabetes. Diabetes Care 2006, 29, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, S.; Rossi, V.; Signorini, C.; Tanganelli, I.; Comporti, M.; Ciccoli, L. Oxidative stress, erythrocyte ageing and plasma non-protein-bound iron in diabetic patients. Free Radic. Res. 2008, 42, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Vinchi, F.; Muckenthaler, M.U.; Da Silva, M.C.; Balla, G.; Balla, J.; Jeney, V. Atherogenesis and iron: From epidemiology to cellular level. Front. Pharmacol. 2014, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Balla, J.; Vercellotti, G.M.; Jeney, V.; Yachie, A.; Varga, Z.; Eaton, J.W.; Balla, G. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol. Nutr. Food Res. 2005, 49, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.R. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematol. Am. Soc. Hematol. Educ. Program 2008, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hess, K. The vulnerable blood. Coagulation and clot structure in diabetes mellitus. Hamostaseologie 2015, 35, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Vinchi, F.; Tolosano, E. Therapeutic approaches to limit hemolysis-driven endothelial dysfunction: Scavenging free heme to preserve vasculature homeostasis. Oxid. Med. Cell. Longev. 2013, 2013, 396527. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D.T.P.; Delattre, J. Free radical and antioxidants (article in French). In Biochimie Pathologique Aspects moléculaires et cellulaires JDelattre, GDurand; Jardillier, J.C., Ed.; Médecine-Sciences: Flammarion, France, 2003; p. 317. [Google Scholar]
- Esposito, K.; Maiorino, M.I.; Petrizzo, M.; Bellastella, G.; Giugliano, D. The effects of a Mediterranean diet on the need for diabetes drugs and remission of newly diagnosed type 2 diabetes: Follow-up of a randomized trial. Diabetes Care 2014, 37, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Bullo, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.R.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Kowluru, V.; Xiong, Y.; Ho, Y.S. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Rad. Biol. Med. 2006, 41, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wada, J.; Hashimoto, I.; Eguchi, J.; Yasuhara, A.; Kanwar, Y.S.; Shikata, K.; Makino, H. Therapeutic approach for diabetic nephropathy using gene delivery of translocase of inner mitochondrial membrane 44 by reducing mitochondrial superoxide production. J. Am. Soc. Nephrol. JASN 2006, 17, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- DeRubertis, F.R.; Craven, P.A.; Melhem, M.F. Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: Effects of tempol. Metabolism 2007, 56, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.M.; Russell, J.W.; Sullivan, K.A.; Backus, C.; Hayes, J.M.; McLean, L.L.; Feldman, E.L. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp. Neurol. 2007, 208, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zheng, S.; Metreveli, N.S.; Epstein, P.N. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 2006, 55, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Di Tomo, P.; Canali, R.; Ciavardelli, D.; di Silvestre, S.; de Marco, A.; Giardinelli, A.; Pipino, C.; di Pietro, N.; Virgili, F.; Pandolfi, A. Beta-Carotene and lycopene affect endothelial response to TNF-alpha reducing nitro-oxidative stress and interaction with monocytes. Mol. Nutr. Food Res. 2012, 56, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J. Nutr. Metab. 2012, 2012, 569486. [Google Scholar] [CrossRef] [PubMed]
- Chanwitheesuk, A.T.A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Dixit, P.P.; Devasagayam, T.P.; Ghaskadbi, S. Formulated antidiabetic preparation Syndrex has a strong antioxidant activity. Eur. J. Pharmacol. 2008, 581, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Qiang, G.; Wenzhai, C.; Huan, Z.; Yuxia, Z.; Dongdong, Y.; Sen, Z.; Qiu, C. Effect of Sancaijiangtang on plasma nitric oxide and endothelin-1 levels in patients with type 2 diabetes mellitus and vascular dementia: A single-blind randomized controlled trial. J. Tradit. Chin. Med. 2015, 35, 375–380. [Google Scholar] [CrossRef]
- Mizutani, K.; Tanaka, O. Stevia, the genus Stevia. Medicinal and aromatic plants—industrial profiles. In Use of Stevia Rebaudiana Sweeteners in Japan; Kinghorn, A.D., Ed.; Taylor and Francis: London, UK, 2002; Volume 19, pp. 178–195. [Google Scholar]
- Kim, J.; Choi, C.H. Stevia, the genus Stevia. Medicinal and aromatic plants—Industrial profiles. In Use of Stevioside and Cultivation; Kinghorn, A.D., Ed.; Departement of medicinal chemistry and pharmacognosy, University of Illinois at Chicago: Chicago, IL, USA, 2002. [Google Scholar]
- Jeppesen, P.B.; Gregersen, S.; Alstrup, K.K.; Hermansen, K. Stevioside induces antihyperglycaemic, insulinotropic and glucagonostatic effectsin vivo: Studies in the diabetic Goto-Kakizaki (GK) rats. Phytomedicine 2002, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, S.; Jeppesen, P.B.; Holst, J.J.; Hermansen, K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism 2004, 53, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Barriocanal, L.A.; Palacios, M.; Benitez, G.; Benitez, S.; Jimenez, J.T.; Jimenez, N.; Rojas, V. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in Type 1 and Type 2 diabetics. Regul. Toxicol. Pharmacol. RTP 2008, 51, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Venkateswaran, S. Protective effect of Coccinia indica on changes in the fatty acid composition in streptozotocin induced diabetic rats. Die Pharm. 2003, 58, 409–412. [Google Scholar]
- Venkateswaran, S.; Pari, L. Effect of Coccinia indica leaf extract on plasma antioxidants in streptozotocin- induced experimental diabetes in rats. Phytother. Res. PTR 2003, 17, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, S.; Pari, L. Effect of Coccinia indica leaves on antioxidant status in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2003, 84, 163–168. [Google Scholar] [CrossRef]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Zhao, C.; Qin, N.; Zhai, H.Y.; Duan, H.Q. Identification of trans-tiliroside as active principle with anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects from Potentilla chinesis. J. Ethnopharmacol. 2011, 135, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Shivanna, N.; Naika, M.; Khanum, F.; Kaul, V.K. Antioxidant, anti-diabetic and renal protective properties of Stevia rebaudiana. J. Diabetes Complicat. 2013, 27, 103–113. [Google Scholar] [CrossRef] [PubMed]
- BelHadj, S.; Hentati, O.; Elfeki, A.; Hamden, K. Inhibitory activities of Ulva lactuca polysaccharides on digestive enzymes related to diabetes and obesity. Arch. Physiol. Biochem. 2013, 119, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Frati Munari, A.C.; Quiroz Lazaro, J.L.; Altamirano Bustamante, P.; Banales Ham, M.; Islas Andrade, S.; Ariza Andraca, C.R. The effect of various doses of nopal (Opuntia streptacantha Lemaire) on the glucose tolerance test in healthy individuals. Archivos de Investigacion Medica 1988, 19, 143–148. [Google Scholar] [PubMed]
- Frati, A.C.; Gordillo, B.E.; Altamirano, P.; Ariza, C.R.; Cortes-Franco, R.; Chavez-Negrete, A. Acute hypoglycemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care 1990, 13, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Frati-Munari, A.C.; Gordillo, B.E.; Altamirano, P.; Ariza, C.R. Hypoglycemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care 1988, 11, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, K.; Hidaka, T.; Nakamura, S.; Umemura, T.; Jitsuiki, D.; Soga, J.; Goto, C.; Chayama, K.; Yoshizumi, M.; Higashi, Y. Pycnogenol, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans. Hypertens. Res. 2007, 30, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Gulati, O.P. Pycnogenol(R) in Metabolic Syndrome and Related Disorders. Phytother. Res. PTR 2015, 29, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Mnafgui, K.; Kchaou, M.; Hamden, K.; Derbali, F.; Slama, S.; Nasri, M.; Salah, H.B.; Allouche, N.; Elfeki, A. Inhibition of carbohydrate and lipid digestive enzymes activities by Zygophyllum album extracts: Effect on blood and pancreas inflammatory biomarkers in alloxan-induced diabetic rats. J. Physiol. Biochem. 2014, 70, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Nomura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. BioFactors 2004, 21, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Serdula, M.; Janket, S.J.; Cook, N.R.; Sesso, H.D.; Willett, W.C.; Manson, J.E.; Buring, J.E. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care 2004, 27, 2993–2996. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H. Fruit and vegetable consumption and diabetes mellitus incidence among U.S. adults. Prev. Med. 2001, 32, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; de la Fuente-Arrillaga, C.; Nunez-Cordoba, J.M.; Basterra-Gortari, F.J.; Beunza, J.J.; Vazquez, Z.; Benito, S.; Tortosa, A.; Bes-Rastrollo, M. Adherence to Mediterranean diet and risk of developing diabetes: Prospective cohort study. BMJ 2008, 336, 1348–1351. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Wilkinson, M.; Penugonda, K.; Simmons, B.; Betts, N.M.; Lyons, T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr. J. 2009, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Burton, W.N.; Chen, C.Y.; Schultz, A.B.; Edington, D.W. The association between achieving low-density lipoprotein cholesterol (LDL-C) goal and statin treatment in an employee population. Popul. Health Manag. 2010, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Upritchard, J.E.; Sutherland, W.H.; Mann, J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Najafian, M.; Jahromi, M.Z.; Nowroznejhad, M.J.; Khajeaian, P.; Kargar, M.M.; Sadeghi, M.; Arasteh, A. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol. Biol. Rep. 2012, 39, 5299–5306. [Google Scholar] [CrossRef] [PubMed]
- Hafizur, R.M.; Kabir, N.; Chishti, S. Asparagus officinalis extract controls blood glucose by improving insulin secretion and beta-cell function in streptozotocin-induced type 2 diabetic rats. Br. J. Nutr. 2012, 108, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, A.; Blazovics, A.; Hagymasi, K.; Kocsis, I.; Kery, A. Antioxidant effect of squeezed juice from black radish (Raphanus sativus L. var niger) in alimentary hyperlipidaemia in rats. Phytother. Res. PTR 2005, 19, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Kar, A. Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside. BioFactors 2007, 31, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Dixit, Y.; Kar, A. Protective role of three vegetable peels in alloxan induced diabetes mellitus in male mice. Plant Foods Hum. Nutr. 2010, 65, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Eidi, A.; Eidi, M.; Esmaeili, E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 2006, 13, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Baluchnejadmojarad, T.; Roghani, M. Garlic extract attenuates time-dependent changes in the reactivity of isolated aorta in streptozotocin-diabetic rats. Life Sci. 2003, 73, 2281–2289. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Yousef, M.I.; Zoheir, M.A. Stannous chloride induces alterations in enzyme activities, lipid peroxidation and histopathology in male rabbit: Antioxidant role of vitamin C. Food Chem. Toxicol. 2005, 43, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Kempaiah, R.K.; Srinivasan, K. Influence of dietary curcumin, capsaicin and garlic on the antioxidant status of red blood cells and the liver in high-fat-fed rats. Ann. Nutr. Metab. 2004, 48, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Venkateswaran, S. Effect of an aqueous extract of Phaseolus vulgaris on the properties of tail tendon collagen of rats with streptozotocin-induced diabetes. Braz. J. Med. Biol. Res. 2003, 36, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.S.; Srinivasan, K. Influence of dietary capsaicin and onion on the metabolic abnormalities associated with streptozotocin induced diabetes mellitus. Mol. Cell. Biochem. 1997, 175, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Kim, J.H.; Choi, H.N.; Kim, M.J.; Han, J.H.; Lee, J.H.; Kim, J.I. Hypoglycemic effects of Welsh onion in an animal model of diabetes mellitus. Nutr. Res. Practice 2010, 4, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Minami, Y.; Ippoushi, K.; Terao, J. Lowering effects of onion intake on oxidative stress biomarkers in streptozotocin-induced diabetic rats. J. Clin. Biochem. Nutr. 2007, 40, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Aoyama, S.; Hamaguchi, N.; Rhi, G.S. Antioxidative and antihypertensive effects of Welsh onion on rats fed with a high-fat high-sucrose diet. Biosci. Biotechnol. Biochem. 2005, 69, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Kataya, H.A.; Hamza, A.A. Red Cabbage (Brassica oleracea) Ameliorates Diabetic Nephropathy in Rats. Evid.-based Complement. Altern. Med. eCAM 2008, 5, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Jalal, R.; Bagheri, S.M.; Moghimi, A.; Rasuli, M.B. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J. Clin. Biochem. Nutr. 2007, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Parelman, M.A.; Storms, D.H.; Kirschke, C.P.; Huang, L.; Zunino, S.J. Dietary strawberry powder reduces blood glucose concentrations in obese and lean C57BL/6 mice, and selectively lowers plasma C-reactive protein in lean mice. Br. J. Nutr. 2012, 108, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Agha, F.G. Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand. J. Clin. Lab. Investig. 2009, 69, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Feelisch, M.; Bjorne, H.; Jansson, E.A.; Weitzberg, E. Cardioprotective effects of vegetables: Is nitrate the answer? Nitric Oxide 2006, 15, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Larsen, F.J.; Nystrom, T.; Hezel, M.; Borniquel, S.; Weitzberg, E.; Lundberg, J.O. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17716–17720. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Benjamin, N. Vegetables and diabetes. Is nitrate the answer? BMJ 2010, 341, c5306. [Google Scholar] [CrossRef] [PubMed]
- Machha, A.; Schechter, A.N. Dietary nitrite and nitrate: A review of potential mechanisms of cardiovascular benefits. Eur. J. Nutr. 2011, 50, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Machha, A.; Schechter, A.N. Inorganic nitrate: A major player in the cardiovascular health benefits of vegetables? Nutr. Rev. 2012, 70, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Cardioprotective actions of nitrite therapy and dietary considerations. Front. Biosci. 2009, 14, 4793–4808. [Google Scholar] [CrossRef]
- Stokes, K.Y.; Dugas, T.R.; Tang, Y.; Garg, H.; Guidry, E.; Bryan, N.S. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1281–H1288. [Google Scholar] [CrossRef] [PubMed]
- Dejam, A.; Hunter, C.J.; Tremonti, C.; Pluta, R.M.; Hon, Y.Y.; Grimes, G.; Partovi, K.; Pelletier, M.M.; Oldfield, E.H.; Cannon, R.O., 3rd; et al. Nitrite infusion in humans and nonhuman primates: Endocrine effects, pharmacokinetics, and tolerance formation. Circulation 2007, 116, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Calvert, J.W.; Gundewar, S.; Lefer, D.J. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Rad. Biol. Med. 2008, 45, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Ekblom, B.; Sahlin, K.; Lundberg, J.O.; Weitzberg, E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006, 355, 2792–2793. [Google Scholar] [CrossRef] [PubMed]
- Timimi, F.K.; Ting, H.H.; Haley, E.A.; Roddy, M.A.; Ganz, P.; Creager, M.A. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1998, 31, 552–557. [Google Scholar] [CrossRef]
- Jain, S.K.; McVie, R.; Jaramillo, J.J.; Palmer, M.; Smith, T.; Meachum, Z.D.; Little, R.L. The effect of modest vitamin E supplementation on lipid peroxidation products and other cardiovascular risk factors in diabetic patients. Lipids 1996, 31 (Suppl), S87–S90. [Google Scholar] [CrossRef] [PubMed]
- Engelen, W.; Keenoy, B.M.; Vertommen, J.; De Leeuw, I. Effects of long-term supplementation with moderate pharmacologic doses of vitamin E are saturable and reversible in patients with type 1 diabetes. Am. J. Clin. Nutr. 2000, 72, 1142–1149. [Google Scholar] [PubMed]
- Heinonen, O.P.; Albanes, D.; Virtamo, J.; Taylor, P.R.; Huttunen, J.K.; Hartman, A.M.; Haapakoski, J.; Malila, N.; Rautalahti, M.; Ripatti, S.; et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: Incidence and mortality in a controlled trial. J. Natl. Cancer Inst. 1998, 90, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.P.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Glynn, R.J.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Sesso, H.D.; Buring, J.E. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2009, 301, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [PubMed]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Sacco, M.; Pellegrini, F.; Roncaglioni, M.C.; Avanzini, F.; Tognoni, G.; Nicolucci, A.; Group PPPC. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: Results of the Primary Prevention Project (PPP) trial. Diabetes Care 2003, 26, 3264–3272. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Dagenais, G.; Pogue, J.; Bosch, J.; Sleight, P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 2000, 342, 154–160. [Google Scholar] [PubMed]
- Gerstein, H.C.; Bosch, J.; Pogue, J.; Taylor, D.W.; Zinman, B.; Yusuf, S. Rationale and design of a large study to evaluate the renal and cardiovascular effects of an ACE inhibitor and vitamin E in high-risk patients with diabetes. The MICRO-HOPE Study. Microalbuminuria, cardiovascular, and renal outcomes. Heart Outcomes Prevention Evaluation. Diabetes Care 1996, 19, 1225–1228. [Google Scholar] [PubMed]
- Blum, S.; Vardi, M.; Brown, J.B.; Russell, A.; Milman, U.; Shapira, C.; Levy, N.S.; Miller-Lotan, R.; Asleh, R.; Levy, A.P. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2–2 genotype. Pharmacogenomics 2010, 11, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Milman, U.; Blum, S.; Shapira, C.; Aronson, D.; Miller-Lotan, R.; Anbinder, Y.; Alshiek, J.; Bennett, L.; Kostenko, M.; Landau, M.; et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2–2 genotype: A prospective double-blinded clinical trial. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Vardi, M.; Blum, S.; Levy, A.P. Haptoglobin genotype and cardiovascular outcomes in diabetes mellitus—Natural history of the disease and the effect of vitamin E treatment. Meta-analysis of the medical literature. Eur. J. Intern. Med. 2012, 23, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; D’Amore, A.; Balbi, V.; Volpe, C.; Galzerano, D.; Giugliano, D.; Sgambato, S.; Varricchio, M.; D'Onofrio, F. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am. J. Physiol. 1994, 266 2 Pt 1, E261–E268. [Google Scholar] [PubMed]
- Chen, H.; Karne, R.J.; Hall, G.; Campia, U.; Panza, J.A.; Cannon, R.O., 3rd; Wang, Y.; Katz, A.; Levine, M.; Quon, M.J. High-dose oral vitamin C partially replenishes vitamin C levels in patients with Type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H137–H145. [Google Scholar] [CrossRef] [PubMed]
- Hirai, N.; Kawano, H.; Hirashima, O.; Motoyama, T.; Moriyama, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Nakao, K.; Yasue, H. Insulin resistance and endothelial dysfunction in smokers: Effects of vitamin C. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1172–H1178. [Google Scholar] [PubMed]
- Kern, T.S.; Engerman, R.L. Comparison of retinal lesions in alloxan-diabetic rats and galactose-fed rats. Curr. Eye Res. 1994, 13, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Hypponen, E.; Laara, E.; Reunanen, A.; Jarvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Stene, L.C.; Joner, G.; Norwegian Childhood Diabetes Study G. Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: A large, population-based, case-control study. Am. J. Clin. Nutr. 2003, 78, 1128–1134. [Google Scholar] [PubMed]
- Kono, K.; Fujii, H.; Nakai, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Shinohara, M.; Hirata, M.; Fukagawa, M.; Nishi, S. Anti-oxidative effect of vitamin D analog on incipient vascular lesion in non-obese type 2 diabetic rats. Am. J. Nephrol. 2013, 37, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, A.; Knekt, P.; Aaran, R.K.; Aromaa, A. Serum antioxidants and risk of non-insulin dependent diabetes mellitus. Eur. J. Clin. Nutr. 1998, 52, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I.; Devaraj, S.; Venugopal, S.K. Oxidative stress, inflammation, and diabetic vasculopathies: The role of alpha tocopherol therapy. Free Rad. Res. 2002, 36, 1331–1336. [Google Scholar] [CrossRef]
- Davie, S.J.; Gould, B.J.; Yudkin, J.S. Effect of vitamin C on glycosylation of proteins. Diabetes 1992, 41, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Giugliano, D.; Quatraro, A.; Donzella, C.; Dipalo, G.; Lefebvre, P.J. Vitamin E reduction of protein glycosylation in diabetes. New prospect for prevention of diabetic complications? Diabetes Care 1991, 14, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Girling, A.J.; Gray, L.; Lunec, J.; Barnett, A.H. An investigation of the relationship between free radical activity and vitamin C metabolism in elderly diabetic subjects with retinopathy. Gerontology 1992, 38, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. Vitamin E improves the action of insulin. Nutr. Rev. 1993, 51, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; D'Amore, A.; Galzerano, D.; Balbi, V.; Giugliano, D.; Varricchio, M.; D'Onofrio, F. Daily vitamin E supplements improve metabolic control but not insulin secretion in elderly type II diabetic patients. Diabetes Care 1993, 16, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; D'Amore, A.; Giugliano, D.; Ceriello, A.; Varricchio, M.; D’Onofrio, F. Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin-dependent diabetic patients. Am. J. Clin. Nutr. 1993, 57, 650–656. [Google Scholar] [PubMed]
- Penckofer, S.; Schwertz, D.; Florczak, K. Oxidative stress and cardiovascular disease in type 2 diabetes: The role of antioxidants and pro-oxidants. J. Cardiovasc. Nurs. 2002, 16, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I. Alpha tocopherol supplementation decreases serum C-reactive protein and monocyte interleukin-6 levels in normal volunteers and type 2 diabetic patients. Free Rad. Biol. Med. 2000, 29, 790–792. [Google Scholar] [CrossRef]
- Neri, S.; Signorelli, S.S.; Torrisi, B.; Pulvirenti, D.; Mauceri, B.; Abate, G.; Ignaccolo, L.; Bordonaro, F.; Cilio, D.; Calvagno, S.; et al. Effects of antioxidant supplementation on postprandial oxidative stress and endothelial dysfunction: A single-blind, 15-day clinical trial in patients with untreated type 2 diabetes, subjects with impaired glucose tolerance, and healthy controls. Clin. Therapeutics 2005, 27, 1764–1773. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.; Vardi, M.; Levy, N.S.; Miller-Lotan, R.; Levy, A.P. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Atherosclerosis 2010, 211, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Bursell, S.E.; Clermont, A.C.; Aiello, L.P.; Aiello, L.M.; Schlossman, D.K.; Feener, E.P.; Laffel, L.; King, G.L. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care 1999, 22, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Bonet, B.; Herrera, E.; Rabano, A. Development of atherosclerosis in the diabetic BALB/c mice. Prevention with Vitamin E administration. Atherosclerosis 2005, 182, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Crino, A.; Schiaffini, R.; Manfrini, S.; Mesturino, C.; Visalli, N.; Anguissola, G.B.; Suraci, C.; Pitocco, D.; Spera, S.; Corbi, S.; et al. A randomized trial of nicotinamide and vitamin E in children with recent onset type 1 diabetes (IMDIAB IX). Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2004, 150, 719–724. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A. The relationship of vascular changes to metabolic factors in diabetes mellitus and their role in the development of peripheral nerve complications. Diabetes/Metab. Rev. 1994, 10, 189–224. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A. Neurovascular dysfunction in diabetic rats. Potential contribution of autoxidation and free radicals examined using transition metal chelating agents. J. Clin. Investig. 1995, 96, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.Q.; Huang, K.X.; Xu, H.B. Influence of alloxan-induced diabetes and selenite treatment on blood glucose and glutathione levels in mice. J. Trace elem. Med. Biol. 2005, 18, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.A. Zinc might protect oxidative changes in the retina and pancreas at the early stage of diabetic rats. Toxicol. Appl. Pharmacol. 2004, 201, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Couthouis, A.; Bertrais, S.; Vergnaud, A.C.; Dauchet, L.; Galan, P.; Hercberg, S. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: Association with dietary intake and plasma concentrations. Am. J. Clin. Nutr. 2006, 84, 395–359. [Google Scholar] [PubMed]
- Maritim, A.; Dene, B.A.; Sanders, R.A.; Watkins, J.B., 3rd. Effects of beta-carotene on oxidative stress in normal and diabetic rats. J. Biochem. Mol. Toxicol. 2002, 16, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Zaltsberg, H.; Ben-Amotz, A.; Kanter, Y.; Aviram, M. Dietary supplementation of a natural isomer mixture of beta-carotene inhibits oxidation of LDL derived from patients with diabetes mellitus. Ann. Nutr. Metab. 2000, 44, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, M.L.; Torronen, R.; Koponen, J.M.; Sinkko, H.; Hellstrom, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [PubMed]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Franzini, L.; Ardigo, D.; Valtuena, S.; Pellegrini, N.; Del Rio, D.; Bianchi, M.A.; Scazzina, F.; Piatti, P.M.; Brighenti, F.; Zavaroni, I. Food selection based on high total antioxidant capacity improves endothelial function in a low cardiovascular risk population. Nutr. Metab. Cardiovasc. Dis. NMCD 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Khymenets, O.; Urpi-Sarda, M.; Tulipani, S.; Garcia-Aloy, M.; Monagas, M.; Mora-Cubillos, X.; Llorach, R.; Andres-Lacueva, C. Cocoa polyphenols and inflammatory markers of cardiovascular disease. Nutrients 2014, 6, 844–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, W.L.; Formanuik, N.L.; Harnpanich, D.; Cheung, M.; Talbot, D.; Chowienczyk, P.J.; Sanders, T.A. A meal enriched with soy isoflavones increases nitric oxide-mediated vasodilation in healthy postmenopausal women. J. Nutr. 2008, 138, 1288–1292. [Google Scholar] [PubMed]
- Boban, M.; Modun, D.; Music, I.; Vukovic, J.; Brizic, I.; Salamunic, I.; Obad, A.; Palada, I.; Dujic, Z. Red wine induced modulation of vascular function: Separating the role of polyphenols, ethanol, and urates. J. Cardiovasc. Pharmacol. 2006, 47, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Agewall, S.; Wright, S.; Doughty, R.N.; Whalley, G.A.; Duxbury, M.; Sharpe, N. Does a glass of red wine improve endothelial function? Eur. Heart J. 2000, 21, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kim, S.; Eto, M.; Iijima, K.; Ako, J.; Yoshizumi, M.; Akishita, M.; Kondo, K.; Itakura, H.; Hosoda, K.; et al. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am. J. Cardiol. 2001, 88, 1457–1460. [Google Scholar] [CrossRef]
- Karatzi, K.; Papamichael, C.; Karatzis, E.; Papaioannou, T.G.; Voidonikola, P.T.; Vamvakou, G.D.; Lekakis, J.; Zampelas, A. Postprandial improvement of endothelial function by red wine and olive oil antioxidants: A synergistic effect of components of the Mediterranean diet. J. Am. Coll. Nutr. 2008, 27, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, C.; Karatzi, K.; Karatzis, E.; Papaioannou, T.G.; Katsichti, P.; Zampelas, A.; Lekakis, J. Combined acute effects of red wine consumption and cigarette smoking on haemodynamics of young smokers. J. Hypertens. 2006, 24, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, C.; Karatzis, E.; Karatzi, K.; Aznaouridis, K.; Papaioannou, T.; Protogerou, A.; Lekakis, J.; Mavrikakis, M. Red wine's antioxidants counteract acute endothelial dysfunction caused by cigarette smoking in healthy nonsmokers. Am. Heart J. 2004, 147, E5. [Google Scholar] [CrossRef]
- Hampton, S.M.; Isherwood, C.; Kirkpatrick, V.J.; Lynne-Smith, A.C.; Griffin, B.A. The influence of alcohol consumed with a meal on endothelial function in healthy individuals. J. Hum. Nutr. Diet. 2010, 23, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, S.R.; Lage, S.H.; Brandizzi, L.; Yoshida, V.; da Luz, P.L. The action of red wine and purple grape juice on vascular reactivity is independent of plasma lipids in hypercholesterolemic patients. Braz. J. Med. Biol. Res. 2005, 38, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.P.; Sutherland, W.H.; McCormick, M.P.; Yeoman, D.J.; de Jong, S.A.; Williams, M.J. Effects of white and red wine on endothelial function in subjects with coronary artery disease. Intern. Med. J. 2004, 34, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Papamichael, C.; Aznaouridis, K.; Karatzis, E.; Lekakis, J.; Matsouka, C.; Boskou, G.; Chiou, A.; Sitara, M.; Feliou, G.; et al. Constituents of red wine other than alcohol improve endothelial function in patients with coronary artery disease. Coron. Artery Dis. 2004, 15, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M. Effect of Grape Seed Extract and Quercetin on Cardiovascular and Endothelial Parameters in High-Risk Subjects. J. Biomed. Biotechnol. 2004, 2004, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekakis, J.; Rallidis, L.S.; Andreadou, I.; Vamvakou, G.; Kazantzoglou, G.; Magiatis, P.; Skaltsounis, A.L.; Kremastinos, D.T. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Keevil, J.G.; Wiebe, D.A.; Aeschlimann, S.; Folts, J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999, 100, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Chou, E.J.; Keevil, J.G.; Aeschlimann, S.; Wiebe, D.A.; Folts, J.D.; Stein, J.H. Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am. J. Cardiol. 2001, 88, 553–555. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, J.S.; Kang, M.H. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: Double-blind, placebo controlled intervention trial. BioFactors 2004, 22, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Kleinbongard, P.; Dejam, A.; Perre, S.; Schroeter, H.; Sies, H.; Kelm, M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005, 46, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Berkels, R.; Roesen, R.; Klaus, W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA 2003, 290, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [PubMed]
- Faridi, Z.; Njike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008, 88, 58–63. [Google Scholar] [PubMed]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005, 81, 611–614. [Google Scholar] [PubMed]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Hall, G.; Kolodziej, T.L.; Karne, R.J.; Crandon, S.K.; Quon, M.J. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am. J. Clin. Nutr. 2008, 88, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Dornfeld, L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001, 158, 195–198. [Google Scholar] [CrossRef]
- Zunino, S.J.; Parelman, M.A.; Freytag, T.L.; Stephensen, C.B.; Kelley, D.S.; Mackey, B.E.; Woodhouse, L.R.; Bonnel, E.L. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br. J. Nutr. 2012, 108, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.J.; Keaney, J.F., Jr.; Holbrook, M.; Gokce, N.; Swerdloff, P.L.; Frei, B.; Vita, J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 2001, 104, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Hamburg, N.M.; Anter, E.; Holbrook, M.; Kahn, D.F.; Elliott, J.G.; Keaney, J.F., Jr.; Vita, J.A. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Nutr. 2007, 26, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fukino, Y.; Shimbo, M.; Aoki, N.; Okubo, T.; Iso, H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J. Nutr. Sci. Vitaminol. 2005, 51, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lobraico, J.M.; DiLello, L.C.; Butler, A.D.; Cordisco, M.E.; Petrini, J.R.; Ahmadi, R. Effects of krill oil on endothelial function and other cardiovascular risk factors in participants with type 2 diabetes, a randomized controlled trial. BMJ Open Diabetes Res. Care 2015, 3, e000107. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Khalili, M.; Haghighat, N.; Eghtesadi, S.; Shidfar, F.; Heidari, I.; Ebrahimpour-Koujan, S.; Eghtesadi, M. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015, 11, 21–29. [Google Scholar] [PubMed]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Hausenblas, H.A.; Schoulda, J.A.; Smoliga, J.M. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus--systematic review and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Wahl, D.; Dieguez, C.; Bernier, M.; de Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.C.; Lokesh, B.R. Studies on the inhibitory effects of curcumin and eugenol on the formation of reactive oxygen species and the oxidation of ferrous iron. Mol. Cell. Biochem. 1994, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ali Hussain, H.E. Hypoglycemic, hypolipidemic and antioxidant properties of combination ofCurcumin fromCurcuma longa, Linn, and partially purified product fromAbroma augusta, Linn. in streptozotocin induced diabetes. Indian J. Clin. Biochem. IJCB 2002, 17, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Rad. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Ryu, O.H.; Lee, J.; Lee, K.W.; Kim, H.Y.; Seo, J.A.; Kim, S.G.; Baik, S.H.; Choi, D.S.; Choi, K.M. Effects of green tea consumption on inflammation, insulin resistance and pulse wave velocity in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2006, 71, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, T.; Leary, L.; Brooks, W.B. The effect of an extract of green and black tea on glucose control in adults with type 2 diabetes mellitus: Double-blind randomized study. Metabolism 2007, 56, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D. Alpha-lipoic acid: A multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol. Therapeutics 2000, 2, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Yin, X.; Lamb, M.M.; Barriga, K.; Seifert, J.; Hoffman, M.; Orton, H.D.; Baron, A.E.; Clare-Salzler, M.; Chase, H.P.S.; et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007, 298, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, B.; Wittmeier, K.; t’Jong, G.W.; McGavock, J.; Robert, M.; Duhamel, T.; Dolinsky, V.W. Proposed trial: Safety and efficacy of resveratrol for the treatment of non-alcoholic fatty liver disease (NAFLD) and associated insulin resistance in adolescents who are overweight or obese adolescents—Rationale and protocol. Biochem. Cell Biol. 2015, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Adibi, P.; Hekmatdoost, A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 114, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Ramazanov, A.; Jimenez Del Rio, M.; Chkhikvishvili, I. Effect of Blueberin on fasting glucose, C-reactive protein and plasma aminotransferases, in female volunteers with diabetes type 2: Double-blind, placebo controlled clinical study. Georgian Med. News 2006, 141, 66–72. [Google Scholar] [PubMed]
- Khan, A.; Safdar, M.; Ali Khan, M.M.; Khattak, K.N.; Anderson, R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.B.; Kuttan, R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J. Physiol. Pharmacol. 1992, 36, 273–275. [Google Scholar] [PubMed]
- van Dam, R.M.; Hu, F.B. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA 2005, 294, 97–104. [Google Scholar] [CrossRef] [PubMed]
- de Munter, J.S.; Hu, F.B.; Spiegelman, D.; Franz, M.; van Dam, R.M. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007, 4, e261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar, P.; Laight, D.; Rooprai, H.K.; Shaw, K.M.; Cummings, M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet. Med. 2009, 26, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Han, G.; Hu, Y.; Bi, Y.; Li, L.; Zhu, D. Tea consumption and risk of type 2 diabetes: A meta-analysis of cohort studies. J. Gen. Intern. Med. 2009, 24, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulos, E.; Zeimbekis, A.; Kastorini, C.M.; Papairakleous, N.; Vlachou, I.; Bountziouka, V.; Panagiotakos, D.B. Effects of black and green tea consumption on blood glucose levels in non-obese elderly men and women from Mediterranean Islands (MEDIS epidemiological study). Eur. J. Nutr. 2008, 47, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Patumraj, S.; Wongeakin, N.; Sridulyakul, P.; Jariyapongskul, A.; Futrakul, N.; Bunnag, S. Combined effects of curcumin and vitamin C to protect endothelial dysfunction in the iris tissue of STZ-induced diabetic rats. Clin. Hemorheol. Microcirc. 2006, 35, 481–489. [Google Scholar] [PubMed]
- Rungseesantivanon, S.; Thenchaisri, N.; Ruangvejvorachai, P.; Patumraj, S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement. Altern. Med. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.I.; Choi, M.S.; Jung, U.J.; Kim, H.J.; Yeo, J.; Jeon, S.M.; Lee, M.K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Rad. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Auberval, N.; Dal, S.; Bietiger, W.; Seyfritz, E.; Peluso, J.; Muller, C.; Zhao, M.; Marchioni, E.; Pinget, M.; Jeandidier, N.; et al. Oxidative Stress Type Influences the Properties of Antioxidants Containing Polyphenols in RINm5F Beta Cells. Evid.-based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. Elmets ca. Green tea polyphenolic antioxidants and skin photoprotection (Review). Int. J. Oncol. 2001, 18, 1307–1313. [Google Scholar] [PubMed]
- Bolling, B.W.; Blumberg, J.B.; Chen, C.Y. Extraction methods determine the antioxidant capacity and induction of quinone reductase by soy products in vitro. Food Chem. 2009, 116, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Song, E.K.; Hur, H.; Han, M.K. Epigallocatechin gallate prevents autoimmune diabetes induced by multiple low doses of streptozotocin in mice. Arch. Pharm. Res. 2003, 26, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.S.; Dugan, C.E.; Smyth, J.A.; DiNatale, D.A.; Koo, S.I. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 2008, 138, 323–331. [Google Scholar] [PubMed]
- Koo, S.I.; Noh, S.K. Green tea as inhibitor of the intestinal absorption of lipids: Potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 2007, 18, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Budin, S.B.; Othman, F.; Louis, S.R.; Bakar, M.A.; Das, S.; Mohamed, J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics 2009, 64, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Blay, M.; Blade, M.C.; Salvado, M.J.; Arola, L.; Ardevol, A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 2004, 145, 4985–4990. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.; Moron, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Su, H.C.; Hung, L.M.; Chen, J.K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1339–E1346. [Google Scholar] [CrossRef] [PubMed]
- Orallo, F.; Alzueta, A.F. Preliminary study of the vasorelaxant effects of (+)-nantenine, an alkaloid isolated from Platycapnos spicata, in rat aorta. Planta Med. 2001, 67, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Ghalib, H.; Modabber, F. Consultation meeting on the development of therapeutic vaccines for post kala azar dermal leishmaniasis. Kinetoplastid Biol. Dis. 2007, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Ouslimani, N.; Peynet, J.; Bonnefont-Rousselot, D.; Therond, P.; Legrand, A.; Beaudeux, J.L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 2005, 54, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Elliott, E.J. Low glycaemic index, or low glycaemic load, diets for diabetes mellitus. Cochrane Database Syst. Rev. 2009, CD006296. [Google Scholar] [CrossRef]
- Nield, L.; Moore, H.J.; Hooper, L.; Cruickshank, J.K.; Vyas, A.; Whittaker, V.; Summerbell, C.D. Dietary advice for treatment of type 2 diabetes mellitus in adults. Cochrane Database Syst. Rev. 2007, CD004097. [Google Scholar] [CrossRef]
- Gaede, P.H.; Jepsen, P.V.; Larsen, J.N.; Jensen, G.V.; Parving, H.H.; Pedersen, O.B. The Steno-2 study. Intensive multifactorial intervention reduces the occurrence of cardiovascular disease in patients with type 2 diabetes. Ugeskrift Laeger 2003, 165, 2658–2661. [Google Scholar]
- Costantino, S.; Paneni, F.; Cosentino, F. Hyperglycemia: A bad signature on the vascular system. Cardiovasc. Diagn. Ther. 2015, 5, 403–406. [Google Scholar] [PubMed]
- Kuschnerus, K.; Landmesser, U.; Krankel, N. Vascular repair strategies in type 2 diabetes: Novel insights. Cardiovasc. Diagn. Ther. 2015, 5, 374–386. [Google Scholar] [PubMed]
- Imamura, F.; O'Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.I.; Story, M.T.; Nelson, M.C. Neighborhood environments: Disparities in access to healthy foods in the U.S. Am. J. Prev. Med. 2009, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Alinia, S.; Hels, O.; Tetens, I. The potential association between fruit intake and body weight—A review. Obes. Rev. 2009, 10, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Chida, Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: Systematic review and meta-analysis. J. Hypertens. 2007, 25, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer—Norfolk prospective study. Arch. Intern. Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L.; Leslie, T.F.; Makara, M.A. Diabetes, obesity, and recommended fruit and vegetable consumption in relation to food environment sub-types: A cross-sectional analysis of Behavioral Risk Factor Surveillance System, United States Census, and food establishment data. BMC Public Health 2015, 15, 491. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Total antioxidant capacity: Appraisal of a concept. J. Nutr. 2007, 137, 1493–1495. [Google Scholar] [PubMed]
- Litescu, S.C.; Eremia, S.; Radu, G.L. Methods for the determination of antioxidant capacity in food and raw materials. Adv. Exp. Med. Biol. 2010, 698, 241–249. [Google Scholar] [PubMed]
- Young, I.S. Measurement of total antioxidant capacity. J. Clin. Pathol. 2001, 54, 339. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.O.; Etienne-Selloum, N.; Dal-Ros, S.; Demougeot, C.; Berthelot, A.; Schini-Kerth, V.B.; Auger, C. Role of arginases in the angiotensin II-induced hypertension-associated endothelial dysfunction in the rat aorta: Preventive effect of red wine polyphenols. J. Phys. Pharm. Adv. 2015, 5, 667–678. [Google Scholar] [CrossRef]
- Kane, M.O.; Etienne-Selloum, N.; Madeira, S.V.; Sarr, M.; Walter, A.; Dal-Ros, S.; Schott, C.; Chataigneau, T.; Schini-Kerth, V.B. Endothelium-derived contracting factors mediate the Ang II-induced endothelial dysfunction in the rat aorta: Preventive effect of red wine polyphenols. Pflugers Arch. 2010, 459, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Auberval, N.; Dal, S.; Maillard, E.; Bietiger, W.; Peronet, C.; Pinget, M.; Schini-Kerth, V.; Sigrist, S. Beneficial effects of a red wine polyphenol extract on high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dal S, S.A.; Maillard-Pedracini, E.; Pinget, M.; Jeandidier, N.; Sigrist, N. Le stress oxydant: Nouvelle cible pour optimiser la prise en charge du patient diabétique de type 1. Infusystems Fr. 2014, 31, 25–30. [Google Scholar]
- Sikand, G.; Kris-Etherton, P.; Boulos, N.M. Impact of functional foods on prevention of cardiovascular disease and diabetes. Curr. Cardiol. Rep. 2015, 17, 39. [Google Scholar] [CrossRef] [PubMed]






| Functional Foods | Bioactive Compound | Mechanisms |
|---|---|---|
| Black tea |
|
|
| Citrus fruit |
|
|
| Dark chocolate |
|
|
| Extravirgin olive oil |
|
|
| Fish |
|
|
| Fruits and vegetables |
|
|
| Ginseng |
|
|
| Grapes and red wine |
|
|
| Green leafy vegetables |
|
|
| Green tea |
|
|
| Margarine |
|
|
| Nuts |
|
|
| Onion and garlic |
|
|
| Pomegranate |
|
|
| Soy proteins |
|
|
| Tomato |
|
|
| Vegetable oil |
|
|
| Whole grains |
|
|
| Plants | Experimental studies | Efficacy |
|---|---|---|
| Allium cepa Allium sativum | Alloxan-induced diabetic rats [277] and STZ-induced diabetic rats [277] | • ROS scavenger • ROS scavenger • ↓ oxidative stress (lipid peroxidation) • ↑ SOD, ↑ GST |
| Aralia elata | STZ-induced diabetic rats [277] | • Inhibition of aldose reductase • Inhibition of cataract (retinopathy) |
| Aloe verra | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) |
| Anoectochilus formosanus | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) |
| Cassia fistula | Alloxan-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) |
| Coccinia indica | STZ-induced diabetic rats [278,279,280] | • hypoglycaemic/hypolipidaemic effects • ↑Vitamin C, antioxidant activity • ↑ antioxidant enzymes activities |
| Eugenia jambolana | STZ-induced diabetic rats [277] | • ROS scavenger |
| Ever green shrubs (Larrea divarita) | STZ-induced diabetic rats [277] | • ↓ XO activity, ion chelation, ROS scavenger, ↓ blood pressure, inhibition of nephropathy |
| Fomes fomentarius | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress biomarkers |
| Juglans regia | T2D-mouse [277] | • ↓ oxidative stress biomarkers |
| Trigonella foenum-graecum (fenugreek) | T2D patients [281] | • Hypoglycemia effect |
| Lycium barbarum | Alloxan-induced diabetic rats [277] | • ↓ lipids |
| Panax ginseng | T2D rats [277] | • ROS scavenger • Erectile dysfunction protection |
| Potentilla chinesis | STZ-induced diabetic rats [282] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) • ↓ blood glucose • ↓ LDL, ↓TG, ↑HDL |
| Scoparia dulcis | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress biomarkers • ↑ GSH |
| Stevia rebaudiana bertoni | STZ-induced diabetic rats [283] | • ↓ blood glucose, ↑glucose tolerance • ↑ insulin levels and ↑sensitivity • ↓ALT, ↓AST, ↑ filtration rate glomerular • Improve kidney damages (nephropathy) • ↓ oxidative stress (lipid peroxidation) • ↑ total antioxidant capacity • ↑ antioxidant enzymes activities |
| Trifolium alexandrium | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress (lipid peroxidation) |
| Ulva lactuca polysaccharides (alga) | STZ-induced diabetic rats [284] | • ↓ blood glucose • ↓enzymes of lipid metabolism and absorption • ↓ LDL, ↓TG, ↑HDL • protection hepatic and renal functions |
| Vitis vinifera | Alloxan-induced diabetic rats [277] | • ROS scavenger • ↑ GSH • ↓ oxidative stress (lipid peroxidation) |
| Viburnim dilatatum | STZ-induced diabetic rats [277] | • ROS scavenger • ↓ oxidative stress (lipid peroxidation) |
| Viscum album | STZ-induced diabetic rats [277] | • ↑ antioxidant enzymes activities • ↓ oxidative stress biomarkers |
| Nopal (Opuntia streptacantha Lemaire) | Healthy people [285] T2D patients [286,287] | • Hypoglycemia effect • ↓ blood glucose, ↓ insulin • ↑ insulin sensitivity |
| Pycnogenol® | Healthy people [288] Hypertensive patients [289] Metabolic syndrome patients [289] | • ↑ NO-mediated forearm blood flow • ↓ blood pressure • Improve endothelial function |
| Zygophyllum album | Alloxan-induced diabetic rats [290] | • ↓ blood glucose, ↓obesity |
| Many plants | STZ-induced diabetic rats [291] | • ion chelation, ROS scavenger • ↓ oxidative stress (lipid peroxidation) |
| Plants like ferula assa-foetida | STZ-induced diabetic rats [277] KK-Ay mice [292] | • ↑ antioxidant enzymes activities • ↓ oxidative stress biomarkers • ↓ blood glucose |
| Fruits or Vegetables | Experimental Studies | Efficacy |
|---|---|---|
| Apple | STZ-induced diabetic rats [300] |
|
| Asparagus | STZ-induced diabetic rats [301] |
|
| Black radish | STZ-induced diabetic ratsHigh Fat Diet rats [302] |
|
| Celery-root | Alloxane-induced diabetic mouse [303] |
|
| Cherry | Alloxane-induced diabetic rats [266] |
|
| Cucumber | Alloxane-induced diabetic mouse [304] |
|
| Garlic | STZ-induced diabetic rats [305,306] |
|
| Alloxane-induced diabetic rats [307] |
| |
| High Fat Diet rats [308] |
| |
| Resistant rats [280] |
| |
| Green bean | STZ-induced diabetic rats [309] |
|
| Onion | STZ-induced diabetic rats [310,311,312] |
|
| High Fat High Sucrose rats [313] |
| |
| Red cabbage | STZ-induced diabetic rats [314] |
|
| Shallot | Fructose-induced Insulin resistant rats [315] |
|
| Strawberry | High Fat Diet mouse [316] |
|
| Tomato | STZ-induced diabetic rats [317] |
|
| Zucchini | Alloxane-induced diabetic mouse [304] |
|
| Vitamins | Human or Experimental Studies | Efficacy |
|---|---|---|
| Vitamin C | T2D patients [343,344] |
|
| T1D patients [329] |
| |
| Healthy patients [343,345] |
| |
| Diabetic rats [346] |
| |
| Vitamin D | Young predisposed child to T1D [347,348] |
|
| T2D-rats [349] |
| |
| Vitamin E | Diabetic patients [350] |
|
| T2D patients [351] |
| |
| T2D patients [352,353] |
| |
| T2D patients [354] |
| |
| T2D patients [355,356,357,358] |
| |
| T2D patients [299,359,360] |
| |
| T2D patients [341,360] |
| |
| Diabetic patients [340,361] |
| |
| T1D patients [330] |
| |
| T1D patients [362] |
| |
| Diabetic Balb/c mice [363] Diabetic rats [346] |
| |
| Combined with nicotinamide | IMDIAB IX study T1D children [331,364] |
|
| Transitional metal chelating agent | STZ-induced diabetic rats [365,366] |
|
| Selenium | Alloxane-induced diabetic rats [367] |
|
| Zinc | STZ-induced diabetic rats [368] |
|
| Combined vitamin C, E, selenium, Zinc and Β-carotene | SU.VI.MAX Healthy patients [369] |
|
| B-carotene | Alloxane-induced diabetic rats [370] and T2D patients [371] |
|
| Natural Sources | Human Studies | Efficacy |
|---|---|---|
| Plants | ||
| Soybean | Woman with CV risk factor [378] | ↑ FMD |
| Grape-derived products | ||
| Red wine + olive oil | Healthy people [379,380,381] Healthy people [382] | ↑ basal FMD ↑ basal FMD |
| Red wine | Atherogenic potential [383,384] Healthy people [385] | ↑ FMD, ↓ blood pressure |
| Hypercholesterolemic patients [386] | improved FMD, enhanced endothelium-independent vasodilation | |
| Coronary artery disease [387,388] | ↑ FMD | |
| Grape juice | Healthy people [389] | ↑ basal FMD |
| Hypercholesterolemic patients [386] | ↑ FMD protect against coronary artery disease | |
| Concord grape juice | Coronary artery disease [390] | ↑ FMD |
| Grape seed extract | Healthy people [391,392] Coronary artery disease [393,394] Hypertensive patients [395] | ↑ basal FMD ↑ FMD ↓ blood pressure |
| Dark chocolate | Atherogenic potential [396] | ↑ basal FMD, ↓ blood pressure |
| Hypertensive patients [397,398] | ↓ blood pressure | |
| Overweight adults [399] | ↑ FMD, ↓ blood pressure (sugar-free preparations) | |
| Healthy people [400] | ↓ blood pressure | |
| Cocoa | patients [401] Overweight adults [399] | ↑ basal FMD by 30% reverse vascular dysfunction no effect on glycaemia control ↑ FMD, ↓ blood pressure(may attenuate by sugar) |
| Hypertensive patients [402] | no effect on blood pressure | |
| Pomegranate juice | Severe carotid artery stenosis [403] Hypertensive patients [404] | ↓ blood pressure, ↓artery thickness ↓ blood pressure |
| Strawberry | Obese patients [405] | ↓ risk factors for CVD and stroke |
| Teas | ||
| Black tea | Coronary artery disease [406] | ↑ FMD |
| EGCG extract (Teavigo®) Green tea | Coronary artery disease [407] | ↑ FMD |
| Borderline diabetes or diabetes [408] | ↓ blood pressure | |
| Healthy prospective cohort [309] | ↓ CV mortality strongly vs. all cause↓ stroke | |
| Coronarien patients [407] | Endothelial cells protection (↑ NO) ↑ FMD | |
| Maritime Pycnogenol® | Healthy people [288] | ↑ NO-mediated forearm blood flow |
| Hypertensive patients [289] | ↓ blood pressure | |
| Metabolic syndrome patients [289] | Improve endothelial function | |
| Oil | ||
| Krill oil (Ѡ3 and fatty acid) | T2D patients [409] | Improve endothelial function ↑ HDL |
| Polyphenols | Human study | Efficacy |
|---|---|---|
| Single compounds | ||
| Quercetin Myricetin | different national public health registers [418] | ↓ risk T2D an chronic disease |
| Quercetin Kaemferol Myricetin Apigenin Luteolin | The Woman’s Health Study [419] | no effect |
| EGCG extract | Overweight or obese men [420] | no effect on insulin sensitivity, no effect on glucose tolerance, modest ↓ in DBP |
| T2D patients [421] | no effect on insulin sensitivity, | |
| T2D patients [408,422] | no effect on HbA1c and glycaemia and Insulin resistance | |
| Lipoic acid | T2D patients [423] | ↑ insulin sensitivity |
| Ѡ-3 | DAISY (Diabetes Autoimmunity Study in the Young) = predisposed T1D-children [424] | ↓ risk of autoimmunity against islets, antioxidant effect |
| Pycnogenol® | Diabetes patients [289] | ↓ blood glucose |
| Hypertensive patients [289] | ↓ blood pressure | |
| Metabolic syndrome patients [289] | ↓ waist circumference, improve lipid profile, renal and endothelial functions | |
| Resveratrol | Diabetes patients [414] | Glucoregulation, ↑ insulin sensitivity, ↑ potency of hypoglycemic agents and antidiabetic therapies |
| Obeses patients [414] | ↑ or↓ insulin sensitivity ↓ adipocyte size ↓ or no effect on circulating inflammatory cytokines ↑ adiponectin | |
| Overweight and obese adolescents [425] | ↓ insulin resistance ↓ non-alcoholic fatty liver disease (NAFLD) | |
| NAFLD patients [426] | no effect on anthropomorphic measurements, insulin markers, lipids profile, blood pressure ↓ NAFLD ↓ALT | |
| Cardiovascular diseases [414] | ↓ or no effect on plasma lipid profile/Chol ↓ systolic blood pressure ↑ Flow-mediated dilatation ↓ pulse-wave velocity | |
| Whole polyphenols diets/foods | ||
| Apple | Middle-age women [419] Men and women [418] | ↓ risk T2D ↓ risk T2D |
| Berry | Men and women [418] | ↓ risk T2D |
| Blueberry | T1D children [308] | ↓ HbA1c, ↑C-peptide, ↑ erythrocyte SOD |
| T2D patients [427] | ↓FBG, ↓ LDL, ↓ CRP ↓ AST, ↓AST, ↓GGT | |
| Cinnamon | T2D patients [428] | ↓ CV risk, ↑ insulin sensitivity |
| Curcumin | Diabetic patients [308] | Improve microangiopathy |
| Healthy people [429] | ↑ HDL, ↓ cholesterol, ↓ lipids peroxidation | |
| Coffee | Metabolic syndrome [430] | ↓ risk T2D |
| Cocoa drink | Hypertensive patients [402] | no effect on insulin resistance no effect on blood pressure |
| Dark chocolate | Healthy people [400] and Hypertensive patients [398] Healthy people [400] | ↑ insulin sensitivity, ↓ blood pressure ↑ QUICKY (insulin sensitivity) ↓ HOMA-IR |
| Whole Grains rich diet | Obesity and T2D patients [431] | ↓ risk T2D |
| Grape seed extract | T2D patients [432] | ↓ glycaemia, ↓ inflammation no effect on HOMA-IR |
| Krill oil (rich inѠ-3) | T2D patients [409] | ↓ blood C-peptide levels, ↓ HOMA-IR, ↑ HDL |
| Purple grape juice | Coronaries patients [393] | ↓ ox LDL |
| Strawberry | Obese patients [405] | ↓ risk factors for CVD and stroke ↓ diabetes |
| Tea | Middle-age women [419] Meta-analysis [433] Non obese people [434] | ↓ risk T2D Prevention of T2D development ↓ risk of obesity, ↓ FBG |
| Green tea | T2D patients [435] Borderline diabetes or diabetes [408] | ↑ levels of insulin ↓ body weight and BMI ↓ blood pressure, ↓blood glucose ↓ HbA1c, ↓HOMA index |
| RWPs – french Corbières AOC | Healthy people [19,20] | ↓ weight, ↓ glycaemia Hypoglycemia effect |
| Polyphenols | Experimental Models | Efficacy |
|---|---|---|
| Curcumin | T2D-rats [436] | ROS scavenger ↓ nephropathy |
| STZ-induced diabetic rats [437] | Protect endothelial dysfunction in the iris : ↓ retinopathy | |
| STZ-induced diabetic rats [438] | Improves mesenteric arteriolar function ↓ ROS artery, ↓ PKC-βII ↓ glycemia | |
| db/db mice [439] | ↓ glycemia, ↓ weight | |
| Ob/ob mice [440] | ↑ glycemic control, ↑insulin sensitivity, ↑ leptin/adiponectin | |
| Bovine aorta [441] | ↓ lipid peroxidation, ROS scavenger | |
| Tea Flavonoids | RINm5f (β-cells) [375] | ROS scavenger Fer and iron scavenger ↓ ROS production |
| Tea EGCG | RINm5f (β-cells) [442] | ↑ mitochondrial activityprotect against oxidative stress ↑ SOD activity ↓ ROS production, ↓ caspase 8 |
| ex vivo skin [443,444] | protection against UV ↑ GSH, ↑ GPx activity | |
| in vitro [445] | prevention of hyperglycemia ↑ insulin activity protection of β cells | |
| STZ-induced diabetes in rats [446] | ↓ β cells lost | |
| (OB/OB) mice [447] | ↓ hepatic steatosis ↓ injury in obese mice | |
| (OB/OB) mice [448] | ↓ intestinal lipid absorption, ↓ body mass, ↓ lipid accumulation in liver and adipocyte, ↑ insulin sensitivity, ↑ TAOC | |
| α lipoic acid | STZ-induced diabetes in rats [449] | ↓ FBG, ↓ HbA1c improve dyslipidemia ↑ SOD activity, ↑endogenous Vit C ↓ MDA and 4-HNE in aorta ↓ DNA damages good vascular morphology |
| Procyanidin B2 (grape seed) | STZ-induced diabetes in rats [450] Β-cells | ↓ plasma glucose Insulin mimetic effect |
| Resveratrol | Zucker fatty (ZF) rats [451] (Obese and T2D) | ↓ T-Chol, ↓ TG |
| STZ-induced T2 diabetes in rats [452] | delay insulin resistance ↓ insulin secretion (hyperinsulinemia) | |
| Endothelial cells of rats [453] | ↓ ROS, ↓NADPH oxidase, ↓inflammation ↓ LDL, antioxidant activity | |
| RWPs extract ProvinolsTM | Zucker fatty (ZF) rats : Obese and T2D [242] | Improve glucose metabolism ↓ plasma glucose, ↓ fructosamine ↓ TG, ↓T-Chol, ↓ LDL Improve cardiac performance (↗ left ventricular and cardiac input) ↓ peripheral arteriole resistances Corrected endothelial dysfunction : in aorta : ↑ NO availability, ↑ NO, ↑ eNOS activity, ↓ O2, ↓ NADPH ox in mesenteric artery : ↑ EDHF |
| RWPs – french Corbières AOC | STZ-induced diabetes in rats and Fructose diet [19,20] | ↓ weight, ↓ glycemia ↓ plasma glucose ↓ plasma lipids |
| RINm5f (β-cells) [442] | ↑ mitochondrial activity protect against oxidative stress ↑ SOD activity ↓ ROS production, ↓ caspase 8 | |
| SOD/CAT mimetics | animal models of diabetic neuropathy [263,264,265] | improve neuropathy |
| translocase of inner mitochondrial membrane | Mice [263] | improve nephropathy |
| tempol | Mice SOD-knockout [264] | improve nephropathy |
| overexpression of MnSOD | Mice [262] | improve retinopathy |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal, S.; Sigrist, S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases 2016, 4, 24. https://doi.org/10.3390/diseases4030024
Dal S, Sigrist S. The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications. Diseases. 2016; 4(3):24. https://doi.org/10.3390/diseases4030024
Chicago/Turabian StyleDal, Stéphanie, and Séverine Sigrist. 2016. "The Protective Effect of Antioxidants Consumption on Diabetes and Vascular Complications" Diseases 4, no. 3: 24. https://doi.org/10.3390/diseases4030024