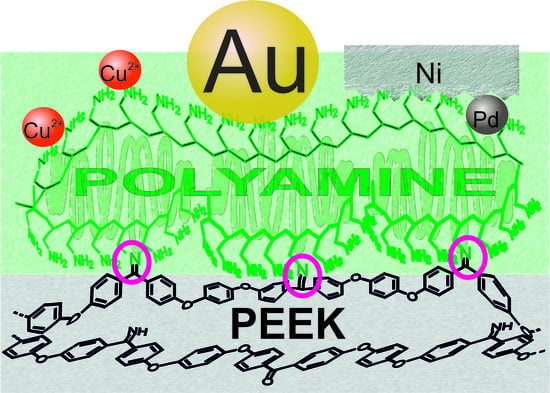
Selective Grafting of Polyamines to Polyether Ether Ketone Surface during Molding and Its Use for Chemical Plating
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laurens, P.; Sadras, B.; Decobert, F.; Arefi-Khonsari, F.; Amouroux, J. Enhancement of the adhesive bonding properties of PEEK by excimer laser treatment. Int. J. Adhes. Adhes. 1998, 18, 19–27. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Kim, H.; Gupta, K.C.; Kang, I.-K. Enhanced tissue compatibility of polyetheretherketone disks by dopamine-mediated protein immobilization. Macromol. Res. 2018, 26, 128–138. [Google Scholar] [CrossRef]
- Kim, K.H.; Im, S.H.; Park, B.J. Long-term stable hydrophilic surface modification of poly(ether ether ketone) via the multilayered chemical grafting method. J. Appl. Polym. Sci. 2018, 135, 46042. [Google Scholar] [CrossRef]
- Rochford, E.T.J.; Poulsson, A.H.C.; Varela, J.S.; Lezuo, P.; Richards, R.G.; Moriarty, T.F. Bacterial adhesion to orthopaedic implant materials and a novel oxygen plasma modified PEEK surface. Colloid Surf. B Biointerfaces 2014, 113, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Al-Maliki, H.; Zsidai, L.; Samyn, P.; Szakal, Z.; Keresztes, R.; Kalacska, G. Effects of atmospheric plasma treatment on adhesion and tribology of aromatic thermoplastic polymers. Polym. Eng. Sci. 2018, 58, E93–E103. [Google Scholar] [CrossRef]
- Salerno, S.; Piscioneri, A.; Laera, S.; Morelli, S.; Favia, P.; Bader, A.; Drioli, E.; De Bartolo, L. Improved functions of human hepatocytes on NH3 plasma-grafted PEEK-WC-PU membranes. Biomaterials 2009, 30, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Stefanikova, R.; Kretkova, T.; Kuzminova, A.; Hanus, J.; Vaidulych, M.; Kylian, O.; Biederman, H. Influence of atmospheric pressure dielectric barrier discharge on wettability and drying of poly(ether-ether-ketone) foils. Polym. Degrad. Stabil. 2018, 150, 114–121. [Google Scholar] [CrossRef]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of oxygen plasma nanotexturing of organic polymer surfaces: From stable super hydrophilic to super hydrophobic surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Meyer-Plath, A.; Keller, D.; Besch, W.; Babucke, G.; Ohl, A. Plasma-induced surface functionalization of polymeric biomaterials in ammonia plasma. Contrib. Plasma Phys. 2001, 41, 562–572. [Google Scholar] [CrossRef]
- Briem, D.; Strametz, S.; Schroder, K.; Meenen, N.M.; Lehmann, W.; Linhart, W.; Ohl, A.; Rueger, J.M. Response of primary fibroblasts and osteoblasts to plasma treated polyetheretherketone (PEEK) surfaces. J. Mater. Sci. Mater. Med. 2005, 16, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, N.; Sureshkumar, A.; Neogi, S. RF plasma-treated polymers for biomedical applications. Curr. Sci. 2008, 94, 1478–1486. [Google Scholar]
- Tsougeni, K.; Papageorgiou, D.; Tserepi, A.; Gogolides, E. “Smart” polymeric microfluidics fabricated by plasma processing: Controlled wetting, capillary filling and hydrophobic valving. Lab Chip 2010, 10, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.W.; Hauert, R.; Ernst, K.H.; Wintermantel, E. Surface analysis of chemically-etched and plasma-treated polyetheretherketone (PEEK) for biomedical applications. Surf. Coat. Technol. 1997, 96, 293–299. [Google Scholar] [CrossRef]
- Iqbal, H.M.S.; Bhowmik, S.; Benedictus, R. Surface modification of high performance polymers by atmospheric pressure plasma and failure mechanism of adhesive bonded joints. Int. J. Adhes. Adhes. 2010, 30, 418–424. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Horiuchi, T.; Suyama, R. Surface modification of poly(aryl ether ether ketone) film by remote oxygen plasma. J. Appl. Polym. Sci. 1998, 68, 271–279. [Google Scholar] [CrossRef]
- Awaja, F.; Cools, P.; Lohberger, B.; Nikiforov, A.Y.; Speranza, G.; Morent, R. Functionalized, biocompatible, and impermeable nanoscale coatings for PEEK. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, I.; Bradley, R.H. Improved adhesion to polymers by UV/ozone surface oxidation. Int. J. Adhes. Adhes. 1996, 16, 29–31. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Gao, H.; Ge, P.; Ren, K.; Gao, J.; Cao, Y.; Han, D.; Zhang, J. One-step fabrication of functionalized poly(etheretherketone) surfaces with enhanced biocompatibility and osteogenic activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Fukazawa, K.; Inoue, Y.; Koyama, J.; Mori, Y.; Kinoshita, T.; Hiranuma, K.; Yasuda, N. Reliable surface modification of dental plastic substrates to reduce biofouling with a photoreactive phospholipid polymer. J. Appl. Polym. Sci. 2018, 135, 46512. [Google Scholar] [CrossRef]
- Rymuszka, D.; Terpiłowski, K.; Borowski, P.; Holysz, L. Time-dependent changes of surface properties of polyether ether ketone caused by air plasma treatment: Air plasma treatment of polyether ether ketone. Polym. Int. 2016, 65, 827–834. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Study of the ageing behaviour of polymer films treated with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7847–7854. [Google Scholar] [CrossRef]
- Noiset, O.; Schneider, Y.J.; Marchand-Brynaert, J. Fibronectin adsorption or and covalent grafting on chemically modified PEEK film surfaces. J. Biomater. Sci. Polym. Ed. 1999, 10, 657–677. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, A.; Soto, R.; Comesana, R.; Boutinguiza, M.; Del Val, J.; Quintero, F.; Lusquinos, F.; Pou, J. Laser surface modification of PEEK. Appl. Surf. Sci. 2012, 258, 9437–9442. [Google Scholar] [CrossRef]
- Rotel, M.; Zahavi, J.; Tamir, S.; Buchman, A.; Dodiuk, H. Pre-bonding technology based on excimer laser surface treatment. Appl. Surf. Sci. 2000, 154, 610–616. [Google Scholar] [CrossRef]
- Lee, K.S.; Shin, M.S.; Lee, J.Y.; Ryu, J.J.; Shin, S.W. Shear bond strength of composite resin to high performance polymer PEKK according to surface treatments and bonding materials. J. Adv. Prosthodont. 2017, 9, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; McCarthy, T.J. Polymer surface modification: Topography effects leading to extreme wettability behavior. Macromolecule 2007, 40, 3965–3969. [Google Scholar] [CrossRef]
- Nagel, J.; Scheidler, D.; Hupfer, B.; Bräuer, M.; Pleul, D.; Vogel, C.; Lehmann, D.; Amesöder, S. Investigations on the formation of composites by injection molding of PA6 and different grafted polypropylenes and their blends. J. Appl. Polym. Sci. 2006, 100, 2992–2999. [Google Scholar] [CrossRef]
- Nagel, J.; Bräuer, M.; Hupfer, B.; Grundke, K.; Schwarz, S.; Lehmann, D. Investigations on the reactive surface modification of polycarbonate by surface-reactive injection molding. J. Appl. Polym. Sci. 2004, 93, 1186–1191. [Google Scholar] [CrossRef]
- Brunotte, R.; Mennig, G.; Nagel, J. In-situ surface modification of polycarbonate during injection moulding. J. Plast. Technol. 2006, 2, 1–7. [Google Scholar]
- Nagel, J.; Brunotte, R.; Hupfer, B.; Grundke, K.; Lehmann, D.; Mennig, G. Investigations into the chemical modification of polyolefin surfaces by radical reactions during molding. Macromol. React. Eng. 2007, 1, 480–487. [Google Scholar] [CrossRef]
- Brunotte, R.; Nagel, J.; Mennig, G.; Heinrich, G.; Gehde, M. Polyolefin surface modification during injection molding using radical reactions in liquid phase. Macromol. React. Eng. 2014, 8, 412–417. [Google Scholar] [CrossRef]
- Nagel, J.; Zimmermann, P.; Schubert, O.; Simon, F.; Schlenstedt, K. Coupling of carboxylic groups onto the surface of polystyrene parts during fused filament fabrication. Appl. Surf. Sci. 2017, 422, 28–31. [Google Scholar] [CrossRef]
- Kroschwald, F.; Nagel, J.; Janke, A.; Simon, F.; Zimmerer, C.; Heinrich, G.; Voit, B. Gold nanoparticle layers from multi-step adsorption immobilised on a polymer surface during injection molding. J. Appl. Polym. Sci. 2016, 133, 43608. [Google Scholar] [CrossRef]
- Ruiz-Cabello, F.M.; Rodríguez-Valverde, M.A.; Cabrerizo-Vilchez, M.A. Contact angle hysteresis on polymer surfaces: An experimental study. J. Adhes. Sci. Technol. 2011, 25, 2039–2049. [Google Scholar] [CrossRef]
- Manolakis, I.; Cross, P.; Colquhoun, H.M. Direct iminization of PEEK. Macromolecules 2011, 44, 7864–7867. [Google Scholar] [CrossRef]
- Yurchenko, M.E.; Huang, J.; Robisson, A.; McKinley, G.H.; Hammond, P.T. Synthesis, mechanical properties and chemical/solvent resistance of crosslinked poly(aryl-ether–ether–ketones) at high temperatures. Polymer 2010, 51, 1914–1920. [Google Scholar] [CrossRef]
- Wright, M.D. Glyoxalated N-Vinylamine. U.S. Patent 8262859 B2, 11 September 2012. [Google Scholar]
- Thompson, S.A.; Farris, R.J. A novel method for crosslinking polyetheretherketone. J. Appl. Polym. Sci. 1988, 36, 1113–1120. [Google Scholar] [CrossRef]
- Becker, M.; Lorenz, S.; Strand, D.; Vahl, C.F.; Gabriel, M. Covalent grafting of the RGD-peptide onto polyetheretherketone surfaces via schiff base formation. Sci. World J. 2013, 2013, 616535. [Google Scholar] [CrossRef] [PubMed]
- Franchina, N.L.; McCarthy, T.J. Surface modifications of poly(ether ether ketone). Macromolecules 1991, 24, 3045–3049. [Google Scholar] [CrossRef]
- Nagel, J.; Heinrich, G. Temperature transitions on the surface of a thermoplastic melt during injection moulding and its use for chemical reactions. Int. J. Heat Mass Transf. 2012, 55, 6890–6896. [Google Scholar] [CrossRef]
- Helfand, E.; Tagami, Y. Theory of the interface between immiscible polymers. J. Polym. Sci. Part B 1971, 9, 741–746. [Google Scholar] [CrossRef]





| Sample Type | Layer Thickness/nm a | Contact Angle/° b | ||
|---|---|---|---|---|
| After Preparation | After Molding | Advancing | Receding | |
| PEEK | – | – | 80 | 52 |
| PEEK-PAc | 36 | 34 | 83 | 52 |
| PEEK-PVA | 18 | 18 | 83 | 53 |
| PEEK-PEI | 115 | 14 | 68 | <10 |
| PEEK-PAAm | 79 | 2 | 84 | <10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagel, J.; Zimmermann, P.; Schwarz, S.; Schlenstedt, K. Selective Grafting of Polyamines to Polyether Ether Ketone Surface during Molding and Its Use for Chemical Plating. Coatings 2018, 8, 333. https://doi.org/10.3390/coatings8100333
Nagel J, Zimmermann P, Schwarz S, Schlenstedt K. Selective Grafting of Polyamines to Polyether Ether Ketone Surface during Molding and Its Use for Chemical Plating. Coatings. 2018; 8(10):333. https://doi.org/10.3390/coatings8100333
Chicago/Turabian StyleNagel, Jürgen, Philipp Zimmermann, Simona Schwarz, and Kornelia Schlenstedt. 2018. "Selective Grafting of Polyamines to Polyether Ether Ketone Surface during Molding and Its Use for Chemical Plating" Coatings 8, no. 10: 333. https://doi.org/10.3390/coatings8100333