Pharmacological Activities of Phytomedicines: A Challenge Horizon for Rational Knowledge
Abstract
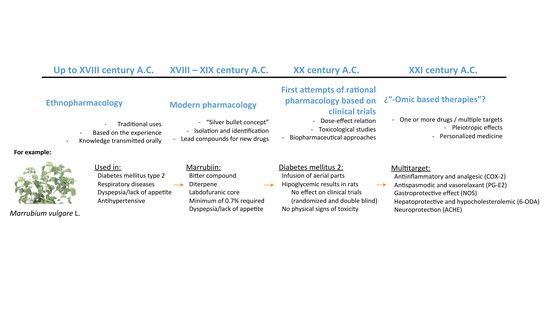
:1. Introduction
2. The “Modern Medicine Approach” Was Not Enough
3. The “-Omic” Technologies; Present and Future
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lietava, J. Medicinal plants in a Middle Paleolithic grave Shanidar IV. J. Ethnoparmacol. 1992, 35, 263–266. [Google Scholar] [CrossRef]
- Sommer, J.D. The Shanidar IV “flower burial”: A re-evaluation of Neanderthal burial ritual. Camb. Archaeol. J. 1999, 9, 127–137. [Google Scholar] [CrossRef]
- Pan, S.Y.; Litscher, G.; Gao, S.H.; Zhou, S.F.; Yu, Z.L.; Chen, H.Q.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ko, K.M.; et al. Historical perspective of traditional indigenous medical practices: The current renaissance and conservation of herbal resources. Evid. Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.C. Trace and Evans, Pharmacognosy, 16th ed.; Churchill Livingstone Elsevier: London, UK, 2009. [Google Scholar]
- Furst, R. Zündorf Evidence-Based Phytotherapy in Europe: Where Do We Stand? Planta Med. 2015, 81, 962–967. [Google Scholar] [PubMed]
- Waller, F. Phytotherapie der traditionellen chinesischen Medizin. Z. Physiother. 1998, 19, 77–89. [Google Scholar]
- Leonti, M.; Verpoort, R. Traditional Mediterranean and European herbal medicines. J. Ethnopharmacol. 2017, 199, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, Y.; Zhou, C.; Qu, H.; Cheng, Y. Discovering active compounds from mixture of natural products by data mining approach. Med. Biol. Eng. Comput. 2008, 46, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Barnes, J.; Gibbons, J.; Williamson, E. Fundamentals of Pharmacognosy and Phytotherapy, 2nd ed.; Churchill Livingstone Elsevier: London, UK, 2012. [Google Scholar]
- Cheng, Y.; Wang, Y.; Wang, X. A causal relationship discovery-based approach to identifying active components of herbal medicine. Comput. Biol. Chem. 2006, 30, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Villanueva, J.; Martin Esteban, J. An Insight into a Blockbuster Phytomedicine; Marrubium vulgare L. Herb. More of a Myth than a Reality? Phytother. Res. 2016, 30, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Borisy, A.A.; Elliott, P.J.; Hurst, N.W.; Lee, M.S.; Lehar, J.; Price, E.R.; Serbedzija, G.; Zimmermann, G.R.; Foley, M.A.; Stockwell, B.R.; et al. Systematic discovery of multicomponent therapeutics. Proc. Natl. Acad. Sci. USA 2003, 100, 7977–7982. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, J.D. Phytochemistry and medicinal plants. Phytochemistry 2001, 56, 237–243. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Merzenich, G.; Zeitler, H.; Jobst, D.; Panek, D.; Vetter, H.; Wagner, H. Application of the “-Omic-” technologies in phytomedicine. Phytomedicine 2007, 14, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Atangwho, I.J.; Ebong, P.E.; Eyong, E.U.; Asmawi, M.Z.; Ahmad, M. Synergistic antidiabetic activity of Vernonia amygdalina and Azadirachta indica: Biochemical effects and possible mechanism. J. Ethnopharmacol. 2012, 141, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Ng, C.H.; Schweitzer, I. ‘Omic’ genetic technologies for herbal medicines in psychiatry. Phytother. Res. 2012, 26, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Shimizu, M.; Ma, H.; Wu, H.A.; Goldsberry, S.; Sicular, S.; Panjikaran, M.; Genovese, G.; Cruz, E. Actein inhibits the Na+-K+-ATPase and enhances the growth inhibitory effect of digitoxin on human breast cancer cells. Biochem. Biophys. Res. Commun. 2008, 375, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; Liao, Z.G.; Zhu, J.Y.; Zhao, G.W.; Yang, M.; Yin, R.L.; Cao, Y.C.; Zhang, J.; Zhao, L.J. The absorption characterization effects and mechanism of Radix Angelicae dahuricae extracts on baicalin in Radix Scutellariae using in vivo and in vitro absorption models. J. Ethnopharmacol. 2012, 139, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10 (Suppl. 1), S4. [Google Scholar] [CrossRef] [PubMed]
- Wegener, T.; Wagner, H. The active components and the pharmacological multi-target principle of STW 5 (Iberogast). Phytomedicine 2006, 13 (Suppl. 5), 20–35. [Google Scholar] [CrossRef] [PubMed]
- Cañigueral, S.; Tschopp, R. The Development of Herbal Medicinal Products. Quality, Safety, and Efficacy as Key Factors. Pharm. Med. 2008, 22, 107–118. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Villanueva, J.; Rodriguez Villanueva, L. Experimental and Clinical Pharmacology of Ziziphus jujuba Mills. Phytother. Res. 2017, 31, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.; Kelber, O.; Lorkowski, G.; Storr, M. Evaluating the Multitarget Effects of Combinations through Multistep Clustering of Pharmacological Data: The Example of the Commercial Preparation Iberogast. Planta Med. 2017, 83, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Ihekwaba, A.E.; Broomhead, D.S.; Grimley, R.; Benson, N.; White, M.R.; Kell, D.B. Synergistic control of oscillations in the NF-kappaB signalling pathway. Syst. Biol. (Stevenage) 2005, 152, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Villanueva, J.; Martin Esteban, J.; Rodríguez Villanueva, L. A Reassessment of the Marrubium vulgare L. Herb’s Potential Role in Diabetes Mellitus Type 2: First Results Guide the Investigation toward New Horizons. Medicines 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, M.; Baudoux, T.; Stévigny, C.; Nortier, J.; Colet, J.M.; Efferth, T.; Qu, F.; Zhou, J.; Chan, K.; Shaw, D.; et al. Review of current and “omics” methods for assessing the toxicity (genotoxicity, teratogenicity and nephrotoxicity) of herbal medicines and mushrooms. J. Ethnopharmacol. 2012, 140, 492–512. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Villanueva, J.; Martin Esteban, J.; Rodríguez Villanueva, L. Solving the puzzle: What is behind our forefathers’ anti-inflammatory remedies? J. Intercult. Ethnopharmacol. 2017, 6, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Nicholson, J.K.; Hylands, P.J.; Sampson, J.; Holmes, E. A metabonomic strategy for the detection of the metabolic effects of chamomile (Matricaria recutita L.) ingestion. J. Agric. Food Chem. 2005, 53, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Merzenich, G.; Panek, D.; Zeitler, H.; Wagner, H.; Vetter, H. New perspectives for synergy research with the “omic”-technologies. Phytomedicine 2009, 16, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Buriani, A.; Garcia-Bermejo, M.L.; Bosisio, E.; Xu, Q.; Li, H.; Dong, X.; Simmonds, M.S.; Carrara, M.; Tejedor, N.; Lucio-Cazana, J.; et al. Omic techniques in systems biology approaches to traditional Chinese medicine research: Present and future. J. Ethnopharmacol. 2012, 140, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Manchikanti, P.; Dey, S. Herbal drugs: Standards and regulation. Fitoterapia 2010, 81, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Claeson, P. Requirements on efficacy of herbal medicinal products. J. Ethnopharmacol. 2014, 158 Pt B, 463–466. [Google Scholar] [CrossRef] [PubMed]
- He, S.M.; Li, C.G.; Liu, J.P.; Chan, E.; Duan, W.; Zhou, S.F. Disposition pathways and pharmacokinetics of herbal medicines in humans. Curr. Med. Chem. 2010, 17, 4072–4113. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Jia, W. An integrated metabolomics and pharmacokinetic strategy for multi-component drugs evaluation. Curr. Drug Metab. 2010, 11, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J. Botanical drugs, synergy, and network pharmacology: Forth and back to intelligent mixtures. Planta Med. 2011, 77, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Noh, K.; Shin, M.; Park, J.; Lee, K.; Nam, H.; Lee, D. In silico profling of systemic efects of drugs to predict unexpected interactions. Nat. Sci. Rep. 2018, 8, 1612. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J. How scientific is the science in ethnopharmacology? Historical perspectives and epistemological problems. J. Ethnopharmacol. 2009, 122, 177–183. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Villanueva, J.; Martín Esteban, J.; Rodríguez Villanueva, L. Pharmacological Activities of Phytomedicines: A Challenge Horizon for Rational Knowledge. Challenges 2018, 9, 15. https://doi.org/10.3390/challe9010015
Rodríguez Villanueva J, Martín Esteban J, Rodríguez Villanueva L. Pharmacological Activities of Phytomedicines: A Challenge Horizon for Rational Knowledge. Challenges. 2018; 9(1):15. https://doi.org/10.3390/challe9010015
Chicago/Turabian StyleRodríguez Villanueva, Javier, Jorge Martín Esteban, and Laura Rodríguez Villanueva. 2018. "Pharmacological Activities of Phytomedicines: A Challenge Horizon for Rational Knowledge" Challenges 9, no. 1: 15. https://doi.org/10.3390/challe9010015