Real-Time Multiphoton Intravital Microscopy of Drug Extravasation in Tumours during Acoustic Cluster Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
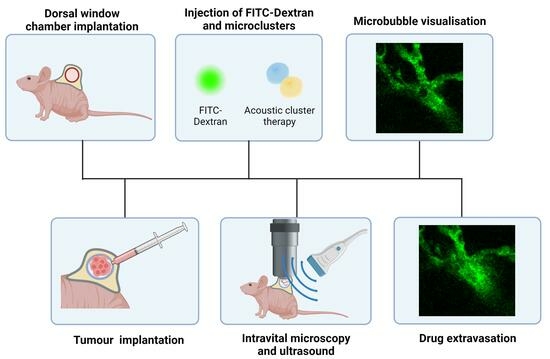
2.2. Tumour Cell Line, Dorsal Windows (DWs), and Tumour Implantations
2.3. Acoustic Cluster Therapy
2.4. Ultrasound Setups
2.5. Co-Alignment of the Acoustic and the Optical Field
2.6. Mouse Holder Design
2.7. Multiphoton Intravital Microscopy (MP-IVM)
2.8. Integrating Ultrasound and Optical Imaging in Live Animal Experiments
- Ultrasound output verification: The post-amplification voltage was verified with an oscilloscope to ensure the correct voltage levels and, thereby, the right acoustic pressure.
- Co-alignment of acoustic and optical fields: The co-alignment was accomplished by positioning the hydrophone’s tip within the optical field of view and then adjusting the transducer’s position by moving the water tank in the XY plane through the movement of the movable stage (Figure 3b) until the hydrophone system detected the peak ultrasound signal.
- MP microscope and sCMOS camera preparation: After alignment, the MP microscope and camera are employed to identify an optimal imaging region. Given that the position of the movable stage with the water tank cannot be changed, the 2D stage placed on the arm of the mouse holder was used to move to a different XY position in the DW chamber.
- Mouse preparation and imaging protocol: The mouse was prepared by inserting a tail vein catheter and injecting dextran-FITC (2 MDa) to visualise the vasculature. Next, the mouse was placed on the mouse holder. Finding a region in the DW where focusing on the vessels was possible was a limiting factor. To overcome this, the strategy was to first, to find an area with a high vascular density by bright field microscopy, no bleeding, and no drainage covering the vessels. Following this, fluorescence microscopy was used to focus on vessels before optimising the focus further when the imaging mode was changed to MP microscopy.
- Selection of the vasculature regions: To increase the probability of visualising microbubbles lodged in the vasculature, the selection of the vasculature regions to be imaged was based on two criteria: first, regions with a dense network of capillaries; and second, vessels similar in size to the activated ACT® microbubbles.
- Treatment: With the ultrasound system on, ACT® microclusters were injected, and the imaging process started for the 5 min and 45 s ACT® protocol. This process was repeated three times per DW: finding a new region, refocusing, re-injecting microclusters, and recording again.
2.9. Image Analysis
2.9.1. Temporal Colour Coding
2.9.2. Distance Map
2.9.3. Penetration Distance
3. Results
3.1. Design of Setups
3.2. Real-Time Visualisation of ACT® Microbubbles
3.3. Visualisation of ACT®-Induced Extravasation Events
3.4. Extravascular Accumulation Dynamics
3.5. Kinetics and Penetration of Dextrans into the Extracellular Matrix (ECM)
4. Discussion
4.1. Experimental Setup for Visualizing ACT® Microbubbles during Ultrasound
4.2. Visualisation of ACT® Microbubbles and Extravasation Events
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anchordoquy, T.J.; Barenholz, Y.; Boraschi, D.; Chorny, M.; Decuzzi, P.; Dobrovolskaia, M.A.; Farhangrazi, Z.S.; Farrell, D.; Gabizon, A.; Ghandehari, H.; et al. Mechanisms and Barriers in Cancer Nanomedicine: Addressing Challenges, Looking for Solutions. ACS Nano 2017, 11, 12–18. [Google Scholar] [CrossRef]
- Tannock, I.F.; Lee, C.M.; Tunggal, J.K.; Cowan, D.S.; Egorin, M.J. Limited penetration of anticancer drugs through tumor tissue: A potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res. 2002, 8, 878–884. [Google Scholar]
- Kim, S.M.; Faix, P.H.; Schnitzer, J.E. Overcoming key biological barriers to cancer drug delivery and efficacy. J. Control. Release 2017, 267, 15–30. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Delord, J.-P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.-A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Aird, W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990, 82, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumor-itropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Boissenot, T.; Bordat, A.; Fattal, E.; Tsapis, N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. J. Control. Release 2016, 241, 144–163. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Voutouri, C.; Mpekris, F.; Stylianopoulos, T. Pancreatic Cancer Presents Distinct Nanomechanical Properties During Progression. Ann. Biomed. Eng. 2023, 51, 1602–1615. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Kotopoulis, S.; Delalande, A.; Popa, M.; Mamaeva, V.; Dimcevski, G.; Gilja, O.H.; Postema, M.; Gjertsen, B.T.; McCormack, E. Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol. Imaging Biol. 2014, 16, 53–62. [Google Scholar] [CrossRef]
- Snipstad, S.; Berg, S.; Mørch, Y.; Bjørkøy, A.; Sulheim, E.; Hansen, R.; Grimstad, I.; van Wamel, A.; Maaland, A.F.; Torp, S.H.; et al. Ultrasound Improves the Delivery and Therapeutic Effect of Nanoparticle-Stabilized Microbubbles in Breast Cancer Xenografts. Ultrasound Med. Biol. 2017, 43, 2651–2669. [Google Scholar] [CrossRef]
- Van Wamel, A.; Sontum, P.C.; Healey, A.; Kvale, S.; Bush, N.; Bamber, J.; de Lange Davies, C. Acoustic Cluster Therapy (ACT) enhances the therapeutic efficacy of paclitaxel and Abraxane(R) for treatment of human prostate adenocarcinoma in mice. J. Control. Release 2016, 236, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wang, Y.; Dong, X.; Lin, Q.; Wang, Y.; Gao, W.; Yun, M.; Li, Y.; Gao, S.; Huang, H.; et al. Clinical sonochemotherapy of inoperable pancreatic cancer using diagnostic ultrasound and microbubbles: A multicentre, open-label, randomised, controlled trial. Eur. Radiol. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re342. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef]
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef]
- Dayton, P.A.; Morgan, K.E.; Klibanov, A.L.; Brandenburger, G.H.; Ferrara, K.W. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1999, 46, 220–232. [Google Scholar] [CrossRef]
- Coussios, C.C.; Roy, R.A. Applications of Acoustics and Cavitation to Noninvasive Therapy and Drug Delivery. Annu. Rev. Fluid Mech. 2008, 40, 395–420. [Google Scholar] [CrossRef]
- Elder, S.A. Cavitation Microstreaming. J. Acoust. Soc. Am. 2005, 31, 54–64. [Google Scholar] [CrossRef]
- Frenkel, V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv. Drug Deliv. Rev. 2008, 60, 1193–1208. [Google Scholar] [CrossRef]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.-M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef]
- Versluis, M.; Stride, E.; Lajoinie, G.; Dollet, B.; Segers, T. Ultrasound Contrast Agent Modeling: A Review. Ultrasound Med. Biol. 2020, 46, 2117–2144. [Google Scholar] [CrossRef] [PubMed]
- Sontum, P.; Kvale, S.; Healey, A.J.; Skurtveit, R.; Watanabe, R.; Matsumura, M.; Ostensen, J. Acoustic Cluster Therapy (ACT)—A novel concept for ultrasound mediated, targeted drug delivery. Int. J. Pharm. 2015, 495, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Healey, A.J.; Sontum, P.C.; Kvåle, S.; Eriksen, M.; Bendiksen, R.; Tornes, A.; Østensen, J. Acoustic Cluster Therapy: In Vitro and Ex Vivo Measurement of Activated Bubble Size Distribution and Temporal Dynamics. Ultrasound Med. Biol. 2016, 42, 1145–1166. [Google Scholar] [CrossRef] [PubMed]
- Van Wamel, A.; Mühlenpfordt, M.; Hansen, R.; Healey, A.; Villanueva, F.S.; Kotopoulis, S.; Davies, C.d.L.; Chen, X. Ultrafast Microscopy Imaging of Acoustic Cluster Therapy Bubbles: Activation and Oscillation. Ultrasound Med. Biol. 2022, 48, 1840–1857. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.; Healey, A.; Shah, A.; Box, G.; Kirkin, V.; Eccles, S.; Sontum, P.C.; Kotopoulis, S.; Kvåle, S.; van Wamel, A.; et al. Theranostic Attributes of Acoustic Cluster Therapy and Its Use for Enhancing the Effectiveness of Liposomal Doxorubicin Treatment of Human Triple Negative Breast Cancer in Mice. Front. Pharmacol. 2020, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Healey, A.J.; Sontum, P.C.; Kvåle, S.; Torp, S.H.; Sulheim, E.; Von Hoff, D.; Han, H. Effect of acoustic cluster therapy (ACT®) combined with chemotherapy in a patient-derived xenograft mouse model of pancreatic cancer. J. Control. Release 2022, 352, 1134–1143. [Google Scholar] [CrossRef]
- Amornphimoltham, P.; Masedunskas, A.; Weigert, R. Intravital microscopy as a tool to study drug delivery in preclinical studies. Adv. Drug Deliv. Rev. 2011, 63, 119–128. [Google Scholar] [CrossRef]
- Hak, S.; Reitan, N.K.; Haraldseth, O.; Davies, C.d.L. Intravital microscopy in window chambers: A unique tool to study tumor angiogenesis and delivery of nanoparticles. Angiogenesis 2010, 13, 113–130. [Google Scholar] [CrossRef]
- Secklehner, J.; Celso, C.L.; Carlin, L.M. Intravital microscopy in historic and contemporary immunology. Immunol. Cell Biol. 2017, 95, 506–513. [Google Scholar] [CrossRef]
- Snipstad, S.; Vikedal, K.; Maardalen, M.; Kurbatskaya, A.; Sulheim, E.; Davies, C.d.L. Ultrasound and microbubbles to beat barriers in tumors: Improving delivery of nanomedicine. Adv. Drug Deliv. Rev. 2021, 177, 113847. [Google Scholar] [CrossRef] [PubMed]
- Momoh, J.; Kapsokalyvas, D.; Vogt, M.; Hak, S.; Kiessling, F.; van Zandvoort, M.; Lammers, T.; Sofias, A.M. Intravital microscopy for real-time monitoring of drug delivery and nanobiological processes. Adv. Drug Deliv. Rev. 2022, 189, 114528. [Google Scholar] [CrossRef] [PubMed]
- Beerling, E.; Ritsma, L.; Vrisekoop, N.; Derksen, P.W.B.; van Rheenen, J. Intravital microscopy: New insights into metastasis of tumors. J. Cell Sci. 2011, 124, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Naumov, G.N.; Wilson, S.M.; MacDonald, I.C.; Schmidt, E.E.; Morris, V.L.; Groom, A.C.; Hoffman, R.M.; Chambers, A.F. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J. Cell Sci. 1999, 112, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.W.; Young, P.A. Principles of Multiphoton Microscopy. Nephron Exp. Nephrol. 2006, 103, e33–e40. [Google Scholar] [CrossRef] [PubMed]
- Holtmaat, A.; Bonhoeffer, T.; Chow, D.K.; Chuckowree, J.; De Paola, V.; Hofer, S.B.; Hübener, M.; Keck, T.; Knott, G.; Lee, W.-C.A.; et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 2009, 4, 1128–1144. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.; Mühlenpfordt, M.; Olsman, M.; Kotopoulis, S.; de Lange Davies, C.; Hynynen, K. Real-time intravital multiphoton microscopy to visualize focused ultrasound and microbubble treatments to increase blood-brain barrier permeability. JoVE 2022, 5, e62235. [Google Scholar]
- Bagher, P.; Segal, S.S. The Mouse Cremaster Muscle Preparation for Intravital Imaging of the Microcirculation. JoVE 2011, 10, e2874. [Google Scholar]
- Tabuchi, A.; Mertens, M.; Kuppe, H.; Pries, A.R.; Kuebler, W.M. Intravital microscopy of the murine pulmonary microcircu-lation. J. Appl. Physiol. 2008, 104, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Dawson, C.A.; Mueller, S.N.; Lindeman, G.J.; Rios, A.C.; Visvader, J.E. Intravital microscopy of dynamic single-cell behavior in mouse mammary tissue. Nat. Protoc. 2021, 16, 1907–1935. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Sorg, B.; Dewhirst, M.W. A novel rodent mammary window of orthotopic breast cancer for intravital microscopy. Microvasc. Res. 2003, 65, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Yemane, P.T.; Åslund, A.K.; Snipstad, S.; Bjørkøy, A.; Grendstad, K.; Berg, S.; Mørch, Y.; Torp, S.H.; Hansen, R.; Davies, C.d.L. Effect of Ultrasound on the Vasculature and Extravasation of Nanoscale Particles Imaged in Real Time. Ultrasound Med. Biol. 2019, 45, 3028–3041. [Google Scholar] [CrossRef]
- Nawijn, C.; Segers, T.; Lajoinie, G.; Morch, Y.; Berg, S.; Snipstad, S.; de Lange Davies, C.; Versluis, M. Multi-timescale Microscopy Methods for the Characterization of Fluorescently-labeled Microbubbles for Ultrasound-Triggered Drug Release. J. Vis. Exp. 2021, e62251. [Google Scholar] [CrossRef]
- Zhao, X.; Pellow, C.; Goertz, D.E. Intravital imaging and cavitation monitoring of antivascular ultrasound in tumor micro-vasculature. Theranostics 2023, 13, 250–266. [Google Scholar] [CrossRef]
- Fodstad, O.; Brøgger, A.; Bruland, O.; Solheim, O.P.; Nesland, J.M.; Pihl, A. Characteristics of a cell line established from a patient with multiple osteosarcoma, appearing 13 years after treatment for bilateral retinoblastoma. Int. J. Cancer 1986, 38, 33–40. [Google Scholar] [CrossRef]
- Andersen, K.K.; Healey, A.; Bush, N.L.; Frijlink, M.E.; Hoff, L. A Harmonic Dual-Frequency Transducer for Acoustic Cluster Therapy. Ultrasound Med. Biol. 2019, 45, 2381–2390. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Masedunskas, A.; Milberg, O.; Porat-Shliom, N.; Sramkova, M.; Wigand, T.; Amornphimoltham, P.; Weigert, R. Intravital microscopy. BioArchitecture 2012, 2, 143–157. [Google Scholar] [CrossRef]
- Mühlenpfordt, M.; Olsen, E.B.; Kotopoulis, S.; Torp, S.H.; Snipstad, S.; Davies, C.d.L.; Olsman, M. Real-Time Intravital Imaging of Acoustic Cluster Therapy–Induced Vascular Effects in the Murine Brain. Ultrasound Med. Biol. 2023, 49, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Nhan, T.; Burgess, A.; Cho, E.E.; Stefanovic, B.; Lilge, L.; Hynynen, K. Drug delivery to the brain by focused ultrasound in-duced blood-brain barrier disruption: Quantitative evaluation of enhanced permeability of cerebral vasculature using two-photon microscopy. J. Control. Release 2013, 172, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Welter, M.; Rieger, H. Physical determinants of vascular network remodeling during tumor growth. Eur. Phys. J. E 2010, 33, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Ferrara, K.W. Acoustic response of compliable microvessels containing ultrasound contrast agents. Phys. Med. Biol. 2006, 51, 5065–5088. [Google Scholar] [CrossRef]
- Myhre, O.; Bjørgan, M.; Grant, D.; Hustvedt, S.; Sontum, P.C.; Dirven, H. Safety assessment in rats and dogs of Acoustic Cluster Therapy, a novel concept for ultrasound mediated, targeted drug delivery. Pharmacol. Res. Perspect. 2016, 4, e00274. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.; Healey, A.; Shah, A.; Box, G.; Kirkin, V.; Kotopoulis, S.; Kvale, S.; Sontum, P.C.; Bamber, J. Therapeutic Dose Response of Acoustic Cluster Therapy in Combination With Irinotecan for the Treatment of Human Colon Cancer in Mice. Front. Pharmacol. 2019, 10, 1299. [Google Scholar] [CrossRef]
- Kooiman, K.; Vos, H.J.; Versluis, M.; de Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.; Lentacker, I. Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The Role of Ultrasound-Driven Microbubble Dynamics in Drug Delivery: From Microbubble Fundamentals to Clinical Translation. Langmuir 2019, 35, 10173–10191. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Lorenz, M.; Poosch, F.; Palme, R.; Zechner, D.; Vollmar, B.; Grambow, E.; Strüder, D. 3D-printed lightweight dorsal skin fold chambers from PEEK reduce chamber-related animal distress. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]










| Ultrasound Parameters | Activation Step | Enhancement Step |
|---|---|---|
| Frequency | 2.7 MHz | 0.5 MHz |
| Peak negative pressure | 400 kPa | 204 kPa |
| Mechanical index | 0.24 | 0.28 |
| Number of cycles | 8 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez, J.L.; Snipstad, S.; Bjørkøy, A.; Davies, C.d.L. Real-Time Multiphoton Intravital Microscopy of Drug Extravasation in Tumours during Acoustic Cluster Therapy. Cells 2024, 13, 349. https://doi.org/10.3390/cells13040349
Fernandez JL, Snipstad S, Bjørkøy A, Davies CdL. Real-Time Multiphoton Intravital Microscopy of Drug Extravasation in Tumours during Acoustic Cluster Therapy. Cells. 2024; 13(4):349. https://doi.org/10.3390/cells13040349
Chicago/Turabian StyleFernandez, Jessica Lage, Sofie Snipstad, Astrid Bjørkøy, and Catharina de Lange Davies. 2024. "Real-Time Multiphoton Intravital Microscopy of Drug Extravasation in Tumours during Acoustic Cluster Therapy" Cells 13, no. 4: 349. https://doi.org/10.3390/cells13040349