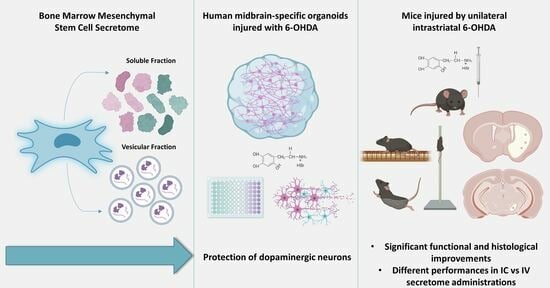
Treating Parkinson’s Disease with Human Bone Marrow Mesenchymal Stem Cell Secretome: A Translational Investigation Using Human Brain Organoids and Different Routes of In Vivo Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. BM-MSC Culture, Secretome Collection and Concentration
2.2. Neuroepithelial Stem Cell Derivation and Generation of Midbrain Organoids
2.3. 6-OHDA Injury and Secretome Treatments In Vitro
2.4. hMO Immunofluorescence Staining
2.5. Image Analysis
2.6. Animals
2.7. Lesion Surgery and Post-Operative Care
2.8. Behavioral Assessment, Secretome Treatments and Experimental Design
2.9. Histological Assessment
2.10. Statistical Analysis
3. Results
3.1. Generation of Human Midbrain-Specific Organoids
3.2. Neuroprotective Effects of BM-MSC Secretome on 6-OHDA-Induced Neurotoxicity in Human Midbrain-Specific Organoids
3.3. BM-MSC Secretome Reverts Parkinsons Disease Motor Symptomatology in a Unilateral Intrastriatal 6-OHDA Model
3.4. Motor Behavior Analysis
3.5. Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Zahoor, I.; Shafi, A.; Haq, E. Pharmacological Treatment of Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Codon Publications: Singapore, 2018; Volume 311, pp. 129–144. ISBN 0098-7484. [Google Scholar]
- Stoker, T.B.; Barker, R.A. Recent Developments in the Treatment of Parkinson’s Disease. F1000Research 2020, 9, 862. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Vizoso, F.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Merimi, M.; El-Majzoub, R.; Lagneaux, L.; Moussa Agha, D.; Bouhtit, F.; Meuleman, N.; Fahmi, H.; Lewalle, P.; Fayyad-Kazan, M.; Najar, M. The Therapeutic Potential of Mesenchymal Stromal Cells for Regenerative Medicine: Current Knowledge and Future Understandings. Front. Cell Dev. Biol. 2021, 9, 661532. [Google Scholar] [CrossRef]
- Marques, C.R.; Marote, A.; Mendes-Pinheiro, B.; Teixeira, F.G.; Salgado, A.J. Cell Secretome Based Approaches in Parkinson’s Disease Regenerative Medicine. Expert Opin. Biol. Ther. 2018, 18, 1235–1245. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Sousa, N.; Salgado, A.J. Mesenchymal Stem Cells Secretome: A New Paradigm for Central Nervous System Regeneration? Cell. Mol. Life Sci. 2013, 70, 3871–3882. [Google Scholar] [CrossRef]
- Pinho, A.G.; Cibrão, J.R.; Silva, N.A.; Monteiro, S.; Salgado, A.J. Cell Secretome: Basic Insights and Therapeutic Opportunities for CNS Disorders. Pharmaceuticals 2020, 13, 31. [Google Scholar] [CrossRef]
- Lázaro, D.F.; Pavlou, M.A.S.; Outeiro, T.F. Cellular Models as Tools for the Study of the Role of Alpha-Synuclein in Parkinson’s Disease. Exp. Neurol. 2017, 298, 162–171. [Google Scholar] [CrossRef]
- Cobb, M.M.; Ravisankar, A.; Skibinski, G.; Finkbeiner, S. iPS Cells in the Study of PD Molecular Pathogenesis. Cell Tissue Res. 2018, 373, 61–77. [Google Scholar] [CrossRef]
- Smits, L.M.; Schwamborn, J.C. Midbrain Organoids: A New Tool to Investigate Parkinson’s Disease. Front. Cell Dev. Biol. 2020, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; van Wuellen, T.; Jarazo, J.; Walter, J.; Brüggemann, I.; Boussaad, I.; et al. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.M.; Reinhardt, L.; Reinhardt, P.; Glatza, M.; Monzel, A.S.; Stanslowsky, N.; Rosato-Siri, M.D.; Zanon, A.; Antony, P.M.; Bellmann, J.; et al. Modeling Parkinson’s Disease in Midbrain-like Organoids. NPJ Park. Dis. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Nickels, S.L.; Modamio, J.; Mendes-Pinheiro, B.; Monzel, A.S.; Betsou, F.; Schwamborn, J.C. Reproducible Generation of Human Midbrain Organoids for in Vitro Modeling of Parkinson’s Disease. Stem Cell Res. 2020, 46, 101870. [Google Scholar] [CrossRef]
- Jarazo, J.; Barmpa, K.; Modamio, J.; Saraiva, C.; Sabaté-Soler, S.; Rosety, I.; Griesbeck, A.; Skwirblies, F.; Zaffaroni, G.; Smits, L.M.; et al. Parkinson’s Disease Phenotypes in Patient Neuronal Cultures and Brain Organoids Improved by 2-Hydroxypropyl-β-Cyclodextrin Treatment. Mov. Disord. 2022, 37, 80–94. [Google Scholar] [CrossRef]
- Zhu, W.; Tao, M.; Hong, Y.; Wu, S.; Chu, C.; Zheng, Z.; Han, X.; Zhu, Q.; Xu, M.; Ewing, A.G.; et al. Dysfunction of Vesicular Storage in Young-Onset Parkinson’s Patient-Derived Dopaminergic Neurons and Organoids Revealed by Single Cell Electrochemical Cytometry. Chem. Sci. 2022, 13, 6217–6223. [Google Scholar] [CrossRef]
- Mohamed, N.-V.; Sirois, J.; Ramamurthy, J.; Mathur, M.; Lépine, P.; Deneault, E.; Maussion, G.; Nicouleau, M.; Chen, C.X.-Q.; Abdian, N.; et al. Midbrain Organoids with an SNCA Gene Triplication Model Key Features of Synucleinopathy. Brain Commun. 2021, 3, fcab223. [Google Scholar] [CrossRef]
- Boussaad, I.; Obermaier, C.D.; Hanss, Z.; Bobbili, D.R.; Bolognin, S.; Glaab, E.; Wołyńska, K.; Weisschuh, N.; De Conti, L.; May, C.; et al. A Patient-Based Model of RNA Mis-Splicing Uncovers Treatment Targets in Parkinson’s Disease. Sci. Transl. Med. 2020, 12, eaau3960. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Panchalingam, K.M.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N.; Salgado, A.J. Impact of the Secretome of Human Mesenchymal Stem Cells on Brain Structure and Animal Behavior in a Rat Model of Parkinson’s Disease. STEM CELLS Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinheiro, B.; Anjo, S.I.; Manadas, B.; Da Silva, J.D.; Marote, A.; Behie, L.A.; Teixeira, F.G.; Salgado, A.J. Bone Marrow Mesenchymal Stem Cells’ Secretome Exerts Neuroprotective Effects in a Parkinson’s Disease Rat Model. Front. Bioeng. Biotechnol. 2019, 7, 294. [Google Scholar] [CrossRef]
- Garcia-Garcia, E.; Andrieux, K.; Gil, S.; Couvreur, P. Colloidal Carriers and Blood-Brain Barrier (BBB) Translocation: A Way to Deliver Drugs to the Brain? Int. J. Pharm. 2005, 298, 274–292. [Google Scholar] [CrossRef]
- Marote, A.; Santos, D.; Mendes-Pinheiro, B.; Serre-Miranda, C.; Anjo, S.I.; Vieira, J.; Ferreira-Antunes, F.; Correia, J.S.; Borges-Pereira, C.; Pinho, A.G.; et al. Cellular Aging Secretes: A Comparison of Bone-Marrow-Derived and Induced Mesenchymal Stem Cells and Their Secretome Over Long-Term Culture. Stem Cell Rev. Rep. 2023, 19, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, P.; Glatza, M.; Hemmer, K.; Tsytsyura, Y.; Thiel, C.S.; Höing, S.; Moritz, S.; Parga, J.A.; Wagner, L.; Bruder, J.M.; et al. Derivation and Expansion Using Only Small Molecules of Human Neural Progenitors for Neurodegenerative Disease Modeling. PLoS ONE 2013, 8, e59252. [Google Scholar] [CrossRef]
- Zagare, A.; Gobin, M.; Monzel, A.S.; Schwamborn, J.C. A Robust Protocol for the Generation of Human Midbrain Organoids. STAR Protoc. 2021, 2, 100524. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.; Morton, D.B.; Burman, O.; Dennison, N.; Honess, P.; Jennings, M.; Lane, S.; Middleton, V.; Roughan, V.J.; Wells, S.; et al. A Guide to Defining and Implementing Protocols for the Welfare Assessment of Laboratory Animals: Eleventh Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 2011, 45, 1–13. [Google Scholar] [CrossRef]
- Mendes-Pinheiro, B.; Soares-Cunha, C.; Marote, A.; Loureiro-Campos, E.; Campos, J.; Barata-Antunes, S.; Monteiro-Fernandes, D.; Santos, D.; Duarte-Silva, S.; Pinto, L.; et al. Unilateral Intrastriatal 6-Hydroxydopamine Lesion in Mice: A Closer Look into Non-Motor Phenotype and Glial Response. Int. J. Mol. Sci. 2021, 22, 11530. [Google Scholar] [CrossRef]
- Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Manadas, B.; Behie, L.A.; Salgado, A.J. Secretome of Undifferentiated Neural Progenitor Cells Induces Histological and Motor Improvements in a Rat Model of Parkinson’s Disease. STEM CELLS Transl. Med. 2018, 7, 829–838. [Google Scholar] [CrossRef]
- Kriegstein, A.; Alvarez-Buylla, A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Harada, A.; Teng, J.; Takei, Y.; Oguchi, K.; Hirokawa, N. MAP2 Is Required for Dendrite Elongation, PKA Anchoring in Dendrites, and Proper PKA Signal Transduction. J Cell Biol 2002, 158, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Armentero, M.T. Animal Models of Parkinson’s Disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Stott, S.R.W.; Barker, R.A. Time Course of Dopamine Neuron Loss and Glial Response in the 6-OHDA Striatal Mouse Model of Parkinson’s Disease. Eur. J. Neurosci. 2014, 39, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Generation of Cerebral Organoids from Human Pluripotent Stem Cells. Nat. Protoc. 2014, 9, 2329–2340. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Domingues, A.V.; Pereira, I.M.; Vilaça-Faria, H.; Salgado, A.J.; Rodrigues, A.J.; Teixeira, F.G. Glial Cells in Parkinson’s Disease: Protective or Deleterious? Cell Mol. Life Sci. 2020, 77, 5171–5188. [Google Scholar] [CrossRef]
- Ni, A.; Ernst, C. Evidence That Substantia Nigra Pars Compacta Dopaminergic Neurons Are Selectively Vulnerable to Oxidative Stress Because They Are Highly Metabolically Active. Front. Cell Neurosci. 2022, 16, 826193. [Google Scholar] [CrossRef]
- Brichta, L.; Greengard, P. Molecular Determinants of Selective Dopaminergic Vulnerability in Parkinson’s Disease: An Update. Front. Neuroanat. 2014, 8, 152. [Google Scholar] [CrossRef]
- Yang, S.-A.; Yoon, J.; Kim, K.; Park, Y. Measurements of Morphological and Biophysical Alterations in Individual Neuron Cells Associated with Early Neurotoxic Effects in Parkinson’s Disease. Cytom. Part A 2017, 91, 510–518. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Jakowec, M.; Burke, R.E.; Przedborski, S. Time Course and Morphology of Dopaminergic Neuronal Death Caused by the Neurotoxin 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine. Neurodegeneration 1995, 4, 257–269. [Google Scholar] [CrossRef]
- Healy-Stoffel, M.; Ahmad, S.O.; Stanford, J.A.; Levant, B. Differential Effects of Intrastriatal 6-Hydroxydopamine on Cell Number and Morphology in Midbrain Dopaminergic Subregions of the Rat. Brain Res. 2014, 1574, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Chen, X.; Oo, T.F.; Kareva, T.; Yarygina, O.; Wang, C.; During, M.; Kholodilov, N.; Burke, R.E. Dopaminergic Pathway Reconstruction by Akt/Rheb-Induced Axon Regeneration. Ann. Neurol. 2011, 70, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and Immune Dysfunction in Parkinson Disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia Integration into Human Midbrain Organoids Leads to Increased Neuronal Maturation and Functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Dong, X.; Chang, L.; Xie, C.; Chang, M.; Aguilar, J.S.; Lin, J.; Lin, J.; Li, Q.Q. Microglia-Containing Cerebral Organoids Derived from Induced Pluripotent Stem Cells for the Study of Neurological Diseases. iScience 2023, 26, 106267. [Google Scholar] [CrossRef]
- Miura, Y.; Li, M.-Y.; Revah, O.; Yoon, S.-J.; Narazaki, G.; Pașca, S.P. Engineering Brain Assembloids to Interrogate Human Neural Circuits. Nat. Protoc. 2022, 17, 15–35. [Google Scholar] [CrossRef]
- Reiner, O.; Sapir, T.; Parichha, A. Using Multi-Organ Culture Systems to Study Parkinson’s Disease. Mol. Psychiatry 2021, 26, 725–735. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Vilaça-Faria, H.; Domingues, A.V.; Campos, J.; Salgado, A.J. Preclinical Comparison of Stem Cells Secretome and Levodopa Application in a 6-Hydroxydopamine Rat Model of Parkinson’s Disease. Cells 2020, 9, 315. [Google Scholar] [CrossRef]
- Vilaça-Faria, H.; Marote, A.; Lages, I.; Ribeiro, C.; Mendes-Pinheiro, B.; Domingues, A.V.; Campos, J.; Lanceros-Mendez, S.; Salgado, A.J.; Teixeira, F.G. Fractionating Stem Cells Secretome for Parkinson’s Disease Modeling: Is It the Whole Better than the Sum of Its Parts? Biochimie 2021, 189, 87–98. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal Models of Parkinson’s Disease: A Source of Novel Treatments and Clues to the Cause of the Disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef]
- Carvalho, M.M.; Campos, F.L.; Coimbra, B.; Pêgo, J.M.; Rodrigues, C.; Lima, R.; Rodrigues, A.J.; Sousa, N.; Salgado, A.J. Behavioral Characterization of the 6-Hydroxidopamine Model of Parkinson’s Disease and Pharmacological Rescuing of Non-Motor Deficits. Mol. Neurodegener. 2013, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishnan, D.; Mohanakumar, K.P. Neuroprotection by Bromocriptine against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Neurotoxicity in Mice1. FASEB J. 1998, 12, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Haobam, R.; Sindhu, K.M.; Chandra, G.; Mohanakumar, K.P. Swim-Test as a Function of Motor Impairment in MPTP Model of Parkinson’s Disease: A Comparative Study in Two Mouse Strains. Behav. Brain Res. 2005, 163, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes Derived from miR-188-3p-Modified Adipose-Derived Mesenchymal Stem Cells Protect Parkinson’s Disease. Mol. Ther. Nucleic Acids 2021, 23, 1334–1344. [Google Scholar] [CrossRef]
- Sun, Z.; Gu, P.; Xu, H.; Zhao, W.; Zhou, Y.; Zhou, L.; Zhang, Z.; Wang, W.; Han, R.; Chai, X.; et al. Human Umbilical Cord Mesenchymal Stem Cells Improve Locomotor Function in Parkinson’s Disease Mouse Model Through Regulating Intestinal Microorganisms. Front. Cell Dev. Biol. 2021, 9, 808905. [Google Scholar] [CrossRef]
- Yildirim, S.; Oylumlu, E.; Ozkan, A.; Sinen, O.; Bulbul, M.; Goksu, E.T.; Ertosun, M.G.; Tanriover, G. Zinc (Zn) and Adipose-Derived Mesenchymal Stem Cells (AD-MSCs) on MPTP-Induced Parkinson’s Disease Model: A Comparative Evaluation of Behavioral and Immunohistochemical Results. NeuroToxicology 2023, 97, 1–11. [Google Scholar] [CrossRef]
- Hortobagyi, T. Beam Walking to Assess Dynamic Balance in Health and Disease: A Protocol for the “BEAM” Multi-Center Observational Study. Available online: https://clinicaltrials.gov/ (accessed on 8 August 2023).
- Kirik, D.; Rosenblad, C.; Björklund, A. Characterization of Behavioral and Neurodegenerative Changes Following Partial Lesions of the Nigrostriatal Dopamine System Induced by Intrastriatal 6-Hydroxydopamine in the Rat. Exp. Neurol. 1998, 152, 259–277. [Google Scholar] [CrossRef]
- Alvarez-Fischer, D.; Henze, C.; Strenzke, C.; Westrich, J.; Ferger, B.; Höglinger, G.U.; Oertel, W.H.; Hartmann, A. Characterization of the Striatal 6-OHDA Model of Parkinson’s Disease in Wild Type and α-Synuclein-Deleted Mice. Exp. Neurol. 2008, 210, 182–193. [Google Scholar] [CrossRef]
- Pires, A.O.; Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Ribeiro-Samy, S.; Gomes, E.D.; Serra, S.C.; Silva, N.A.; Manadas, B.; Sousa, N.; et al. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue-Derived Stem Cells, and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016, 25, 1073–1083. [Google Scholar] [CrossRef]
- Marques, C.R.; Pereira-Sousa, J.; Teixeira, F.G.; Sousa, R.A.; Teixeira-Castro, A.; Salgado, A.J. Mesenchymal Stem Cell Secretome Protects against Alpha-Synuclein-Induced Neurodegeneration in a Caenorhabditis Elegans Model of Parkinson’s Disease. Cytotherapy 2021, 10, 894–901. [Google Scholar] [CrossRef]
- Marques, C.R.; Fuzeta, M. de A.; dos Santos Cunha, R.M.; Pereira-Sousa, J.; Silva, D.; Campos, J.; Teixeira-Castro, A.; Sousa, R.A.; Fernandes-Platzgummer, A.; da Silva, C.L.; et al. Neurodifferentiation and Neuroprotection Potential of Mesenchymal Stromal Cell-Derived Secretome Produced in Different Dynamic Systems. Biomedicines 2023, 11, 1240. [Google Scholar] [CrossRef]
- Lev, N.; Barhum, Y.; Ben-Zur, T.; Melamed, E.; Steiner, I.; Offen, D. Knocking out DJ-1 Attenuates Astrocytes Neuroprotection against 6-Hydroxydopamine Toxicity. J. Mol. Neurosci. 2013, 50, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, H.N.; Park, H.J.; Shin, J.Y.; Kim, D.Y.; Lee, P.H. The Cleavage Effect of Mesenchymal Stem Cell and Its Derived Matrix Metalloproteinase-2 on Extracellular α-Synuclein Aggregates in Parkinsonian Models. STEM CELLS Transl. Med. 2017, 6, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kobayashi, Y.; Inoshita, T.; Meng, H.; Arano, T.; Uemura, K.; Asano, T.; Yoshimi, K.; Zhang, C.-L.; Matsumoto, G.; et al. The Parkinson’s Disease-Associated Protein Kinase LRRK2 Modulates Notch Signaling through the Endosomal Pathway. PLoS Genet 2015, 11, e1005503. [Google Scholar] [CrossRef] [PubMed]
- Desplats, P.; Spencer, B.; Crews, L.; Pathel, P.; Morvinski-Friedmann, D.; Kosberg, K.; Roberts, S.; Patrick, C.; Winner, B.; Winkler, J.; et al. α-Synuclein Induces Alterations in Adult Neurogenesis in Parkinson Disease Models via P53-Mediated Repression of Notch1. J. Biol. Chem. 2012, 287, 31691–31702. [Google Scholar] [CrossRef] [PubMed]
- Marote, A.; Teixeira, F.G.; Mendes-Pinheiro, B.; Salgado, A.J. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front. Pharmacol. 2016, 7, 231. [Google Scholar] [CrossRef]
- Di Santo, S.; Seiler, S.; Ducray, A.D.; Widmer, H.R. Conditioned Medium from Endothelial Progenitor Cells Promotes Number of Dopaminergic Neurons and Exerts Neuroprotection in Cultured Ventral Mesencephalic Neuronal Progenitor Cells. Brain Res. 2019, 1720, 146330. [Google Scholar] [CrossRef]
- Ni, W.; Zhou, J.; Ling, Y.; Lu, X.; Niu, D.; Zeng, Y.; Qiu, Y.; Si, Y.; Wang, J.; Zhang, W.; et al. Neural Stem Cell Secretome Exerts a Protective Effect on Damaged Neuron Mitochondria in Parkinson’s Disease Model. Brain Res. 2022, 1790, 147978. [Google Scholar] [CrossRef]
- Monzel, A.S.; Hemmer, K.; Kaoma, T.; Smits, L.M.; Bolognin, S.; Lucarelli, P.; Rosety, I.; Zagare, A.; Antony, P.; Nickels, S.L.; et al. Machine Learning-Assisted Neurotoxicity Prediction in Human Midbrain Organoids. Park. Relat. Disord. 2020, 75, 105–109. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes-Pinheiro, B.; Campos, J.; Marote, A.; Soares-Cunha, C.; Nickels, S.L.; Monzel, A.S.; Cibrão, J.R.; Loureiro-Campos, E.; Serra, S.C.; Barata-Antunes, S.; et al. Treating Parkinson’s Disease with Human Bone Marrow Mesenchymal Stem Cell Secretome: A Translational Investigation Using Human Brain Organoids and Different Routes of In Vivo Administration. Cells 2023, 12, 2565. https://doi.org/10.3390/cells12212565
Mendes-Pinheiro B, Campos J, Marote A, Soares-Cunha C, Nickels SL, Monzel AS, Cibrão JR, Loureiro-Campos E, Serra SC, Barata-Antunes S, et al. Treating Parkinson’s Disease with Human Bone Marrow Mesenchymal Stem Cell Secretome: A Translational Investigation Using Human Brain Organoids and Different Routes of In Vivo Administration. Cells. 2023; 12(21):2565. https://doi.org/10.3390/cells12212565
Chicago/Turabian StyleMendes-Pinheiro, Bárbara, Jonas Campos, Ana Marote, Carina Soares-Cunha, Sarah L. Nickels, Anna S. Monzel, Jorge R. Cibrão, Eduardo Loureiro-Campos, Sofia C. Serra, Sandra Barata-Antunes, and et al. 2023. "Treating Parkinson’s Disease with Human Bone Marrow Mesenchymal Stem Cell Secretome: A Translational Investigation Using Human Brain Organoids and Different Routes of In Vivo Administration" Cells 12, no. 21: 2565. https://doi.org/10.3390/cells12212565