Radiative and Non-Radiative Recombination Pathways in Mixed-Phase TiO2 Nanotubes for PEC Water-Splitting
Abstract
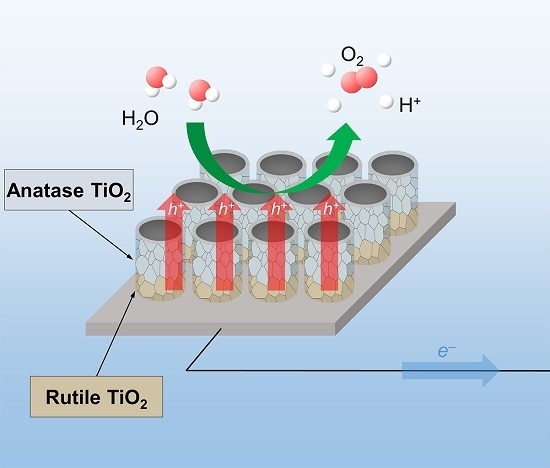
:1. Introduction
2. Results and Discussion
2.1. Structural and Optical Properties
2.2. Photoelectrochemical Measurements
2.3. Intensity-Modulated Photocurrent Spectroscopy (IMPS)
2.4. Intensity-Modulated Photovoltage Spectroscopy (IMVS)
2.5. Electrochemical Impedance Spectroscopy (EIS)
3. Materials and Methods
3.1. Characterization
3.2. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boddy, P.J. Oxygen evolution on semiconducting TiO2. J. Electrochem. Soc. 1968, 115, 199–203. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Krol, R.V.; Liang, Y.; Schoonman, J. Solar hydrogen production with nanostructured metal oxides. J. Mater. Chem. 2008, 18, 2311–2320. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Shwarsctein, A.K.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photo-electrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Sivula, K.; Krol, R.V. Semiconducting materials for photo-electrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Kment, S.; Schmuki, P.; Hubicka, Z.; Machala, L.; Kirchgeorg, R.; Liu, N.; Wang, L.; Lee, K.; Olejnicek, J.; Cada, M.; et al. Photoanodes with fully controllable texture: The enhanced water-splitting efficiency of thin hematite films exhibiting solely (110) crystal orientation. ACS Nano 2015, 9, 7113–7123. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kweon, K.E.; Ye, H.; Paek, E.; Hwang, G.S.; Bard, A.J. Factors in the metal doping of BiVO4 for improved photoelectrocatalytic activity as studied by scanning electrochemical microscopy and first-principles density-functional calculation. J. Phys. Chem. C 2011, 115, 17870–17879. [Google Scholar] [CrossRef]
- Wang, J.; Tafen, D.N.; Lewis, J.P.; Hong, Z.; Manivannan, A.; Zhi, M.; Li, M.; Wu, N. Origin of photocatalytic activity of nitrogen-doped TiO2 nanobelts. J. Am. Chem. Soc. 2009, 131, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Akurati, K.K.; Fortunato, G.; Reifler, F.A.; Ritter, A.; Harvey, A.S.; Vital, A.; Graule, T. Nitrogen doping of TiO2 photocatalyst forms a second eg state in the oxygen 1s NEXAFS pre-edge. J. Phys. Chem. C 2010, 114, 516–519. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B., Jr. Efficient photochemical water-splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, J.; Chen, H.M.; Liu, B.; Yang, P. A Fully integrated nanosystem of semiconductor nanowires for direct solar water-splitting. Nano Lett. 2013, 13, 2989–2992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, K.; Han, J.; Li, Y.; Cui, T.; Wang, B.; Ya, J.; Zhou, C. Dendritic TiO2/ln2S3/AgInS2 trilaminar core-shell branched nanoarrays and the enhanced activity for photo-electrochemical water-splitting. Small 2014, 10, 3153–3161. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Mori, T.; Watanabe, M. Influence of platinum loading on photoluminescence of TiO2 powder. J. Appl. Phys. 2004, 96, 925. [Google Scholar] [CrossRef]
- Wu, G.P.; Chen, T.; Su, W.G.; Zhou, G.H.; Zong, X.; Lei, Z.B.; Li, C. H2 production with ultra-low CO selectivity via photocatalytic reforming of methanol on Au/TiO2 catalyst. Int. J. Hydrogen Energy 2008, 33, 1243–1251. [Google Scholar] [CrossRef]
- Malara, F.; Minguzzi, A.; Marelli, M.; Morandi, S.; Psaro, R.; Dal Santo, V.; Naldoni, A. α-Fe2O3/NiOOH: An Effective heterostructure for photoelectrochemical water oxidation. ACS Catal. 2015, 5, 5292–5300. [Google Scholar] [CrossRef]
- Cai, H.; Yang, Q.; Hu, Z.; Zhihua, D.; You, Q.; Sun, J.; Xu, N.; Wu, J. Enhanced photoelectrochemical activity of vertically aligned ZnO-coated TiO2 nanotubes. Appl. Phys. Lett. 2014, 104, 053114. [Google Scholar] [CrossRef]
- Gui, Q.; Xu, Z.; Zhang, H.; Cheng, C.; Zhu, X.; Yin, M.; Song, Y.; Lu, L.; Chen, X.; Li, D. Enhanced photoelectrochemical water-splitting performance of anodic TiO2 nanotube arrays by surface passivation. ACS Appl. Mater. Interfaces 2014, 6, 17053–17058. [Google Scholar] [CrossRef] [PubMed]
- Sopha, H.; Krbal, M.; Ng, S.; Prikryl, J.; Zazpe, R.; Yam, F.K.; Macak, J.M. One-dimensional anodic TiO2 nanotubes coated by atomic layer deposition: Towards advanced applications. Appl. Mater. Today 2017, 9, 104–110. [Google Scholar] [CrossRef]
- Kavan, L.; Gratzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and photoelectrochemical investigation of single-crystal anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Naldoni, A.; Montini, T.; Malara, F.; Mroz, M.M.; Beltram, A.; Virgili, T.; Boldrini, C.L.; Marelli, M.; Romero-Ocaña, I.; Delgado, J.J.; et al. Hot electron collection on brookite nanorods lateral facets for plasmon-enhanced water oxidation. ACS Catal. 2017, 7, 1270–1278. [Google Scholar] [CrossRef]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X. Branched TiO2 nanorods for photoelectrochemical hydrogen production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water-splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef] [PubMed]
- Altomare, M.; Nguyen, N.T.; Hejazi, S.; Schmuki, P. A Cocatalytic electron-transfer cascade site-selectively placed on TiO2 nanotubes yields enhanced photocatalytic H2 Evolution. Adv. Funct. Mater. 2018, 8, 1704259. [Google Scholar] [CrossRef]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J. A structural investigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Komaguchi, K.; Nakano, H.; Araki, A.; Harima, Y. Photoinduced electron transfer from anatase to rutile in partially reduced TiO2 (P-25) nanoparticles: An ESR study. Chem. Phys. Lett. 2016, 428, 338–342. [Google Scholar] [CrossRef]
- Kang, J.; Wu, F.; Li, S.-S.; Xia, J.B.; Li, J. Calculating band alignment between materials with different structures: The case of anatase and rutile titanium dioxide. J. Phys. Chem. C 2012, 116, 20765–20768. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2013, 107, 4545–4549. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Reconsideration of intrinsic band alignments within anatase and rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Kullgren, J.; Aradi, B.; Frauenheim, T.; Kavan, L.; Deak, P. Resolving the controversy about the band alignment between Rutile and Anatase: The role of OH−/H+ adsorption. J. Phys. Chem. C 2015, 119, 21952–21958. [Google Scholar] [CrossRef]
- Li, A.; Wang, Z.; Yin, H.; Wang, S.; Yan, P.; Huang, B.; Wang, X.; Li, R.; Zong, X.; Han, H.; et al. Understanding the anatase-rutile phase junction in charge separation and transfer in a TiO2 electrode for photoelectrochemical water-splitting. Chem. Sci. 2016, 7, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Pena, P.A.; Carrera Crespo, J.E.; Gonzalez, F.; Gonzalez, I. Effect of heat treatment on the crystal phase composition, semiconducting properties and photoelectrocatalytic color removal efficiency of TiO2 nanotubes arrays. Electrochimica. Acta 2014, 140, 564–571. [Google Scholar] [CrossRef]
- Regonini, D.; Jaroenworaluck, A.; Stevens, R.; Bowen, C.R. Effect of heat treatment on the properties and structure of TiO2 nanotubes: Phase composition and chemical composition. Surf. Interface Anal. 2010, 42, 139–144. [Google Scholar] [CrossRef]
- Ponomarev, E.A.; Peter, L.M. A generalized theory of intensity modulated photocurrent spectroscopy (IMPS). J. Electroanal. Chem. 1995, 396, 219–226. [Google Scholar] [CrossRef]
- Cachet, H.; Sutter, E.M.M. Kinetics of water oxidation at TiO2 nanotube arrays at different pH domains investigated by electrochemical and light-modulated impedance Spectroscopy. J. Phys. Chem. C 2015, 119, 25548–25558. [Google Scholar] [CrossRef]
- Klotz, D.; Ellis, D.S.; Dotan, H.; Rothschild, A. Empirical in operando analysis of the charge carrier dynamics in hematite photoanodes by PEIS, IMPS and IMVS. Phys. Chem. Chem. Phys. 2016, 18, 23438–23457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottineau, T.; Cachet, H.; Keller, V.; Sutter, E.M.M. Influence of the anatase/rutile ratio on the charge transport properties of TiO2-NTs arrays studied by dual wavelength opto-electrochemical impedance spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 31469–31478. [Google Scholar] [CrossRef] [PubMed]
- Riboni, F.; Nguyen, N.T.; So, S.; Schmuki, P. Aligned metal oxide nanotube arrays: Key-aspects of anodic TiO2 nanotube formation and properties. Nanoscale Horiz. 2016, 1, 445–466. [Google Scholar] [CrossRef]
- Ampelli, C.; Tavella, F.; Perathoner, S.; Centi, G. Engineering of photoanodes based on ordered TiO2-nanotube arrays in solar photo-electrocatalytic (PECa) cells. Chem. Eng. J. 2017, 320, 352–362. [Google Scholar] [CrossRef]
- Ampelli, C.; Tavella, F.; Genovese, C.; Perathoner, S.; Favaro, M.; Centi, G. Analysis of the factors controlling performances of Au-modified TiO2 nanotube array based photoanode in photo-electrocatalytic (PECa) cells. J. Energy Chem. 2017, 26, 284–294. [Google Scholar] [CrossRef]
- Saboo, T.; Tavella, F.; Ampelli, C.; Perathoner, S.; Genovese, C.; Marepally, B.C.; Veyre, L.; Elsje, A.Q.; Centi, G. Water-splitting on 3D-type meso/macro porous structured photoanodes based on Ti mesh. Sol. Energy Mater. Sol. Cells 2018, 98, 178. [Google Scholar] [CrossRef]
- Albu, S.P.; Tsuchiya, H.; Fujimoto, S.; Schmuki, P. TiO2 Nanotubes-annealing effects on detailed morphology and structure. Eur. J. Inorg. Chem. 2010, 2010, 4351–4356. [Google Scholar] [CrossRef]
- Mohammadpour, F.; Altomare, M.; So, S.; Lee, K.; Mokhtar, M.; Alshehri, A.; Al-Thabaiti, S.A.; Schmuki, P. High-temperature annealing of TiO2 nanotube membranes for efficient dye-sensitized solar cells. Semicond. Sci. Technol. 2016, 31, 014010. [Google Scholar] [CrossRef]
- Yoo, J.E.; Altomare, M.; Mokhtar, M.; Alshehri, A.; Al-Thabaiti, S.A.; Mazare, A.; Schmuki, P. Photocatalytic H2 generation using dewetted Pt-decorated TiO2 nanotubes: Optimized dewetting and oxide crystallization by a multiple annealing process. J. Phys. Chem. C 2016, 120, 15884–15892. [Google Scholar] [CrossRef]
- Jarosz, M.; Syrek, K.; Kapusta-Kołodziej, J.; Mech, J.; Malek, K.; Hnida, K.; Lojewski, T.; Jaskula, M.; Sulka, G.D. Heat treatment effect on crystalline structure and photo-electrochemical properties of anodic TiO2 nanotube arrays formed in ethylene glycol and glycerol based electrolytes. J. Phys. Chem. C 2015, 119, 24182–24191. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Wang, J.; Sham, T.K. Unfolding the Anatase-to-Rutile phase transition in TiO2 nanotubes using X-ray spectroscopy and spectromicroscopy. J. Phys. Chem. C 2016, 120, 22079–22087. [Google Scholar] [CrossRef]
- Scaramuzzo, F.A.; Dell’Era, A.; Tarquini, G.; Caminiti, R.; Ballirano, P.; Pasquali, M. Phase transition of TiO2 nanotubes: An X-ray study as a function of temperature. J. Phys. Chem. C 2017, 121, 24871–24876. [Google Scholar] [CrossRef]
- Li, W.; Ni, C.; Lin, H.; Huang, C.P.; Shah, S.I. Size dependence of thermal stability of TiO2 nanoparticles. J. Appl. Phys. 2004, 96, 6663–6668. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Santo, V.D. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Likodimos, V.; Stergiopoulos, T.; Falaras, P.; Kunze, J.; Schmuki, P. Phase composition, size, orientation, and antenna effects of self-assembled anodized titania nanotube arrays: A polarized micro-raman investigation. J. Phys. Chem. C 2008, 112, 12687–12696. [Google Scholar] [CrossRef]
- Rambabu, Y.; Jaiswal, M.; Roy, S.C. Effect of annealing temperature on the phase transition, structural stability and photo-electrochemical performance of TiO2 multi-leg nanotubes. Catal. Today 2016, 278, 255–261. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Li Bassi, A.; Cattaneo, D.; Russo, V.; Bottani, C.E. Raman spectroscopy characterization of titania nanoparticles produced by flame pyrolysis: The influence of size and stoichiometry. J. Appl. Phys. 2005, 98, 074305. [Google Scholar] [CrossRef]
- Knorr, F.J.; Mercado, C.C.; McHale, J.L. Trap-state distributions and carrier transport in pure and mixed-phase TiO2: Influence of contacting solvent and interphasial electron transfer. J. Phys. Chem. C 2008, 112, 12786–12794. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Feng, Z.; Chen, T.; Lian, Y.; Wang, X.; Li, C. Photoluminescence Characteristics of TiO2 and Their Relationship to the photoassisted reaction of Water/Methanol Mixture. J. Phys. Chem. C 2017, 111, 693–699. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Shi, J.; Jia, G.; Shen, S.; Zhou, J.; Li, C. Trap states and carrier dynamics of TiO2 studied by photoluminescence spectroscopy under weak excitation condition. Phys. Chem. Chem. Phys. 2010, 12, 7083–7090. [Google Scholar] [CrossRef] [PubMed]
- Pallotti, D.K.; Passoni, L.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Photoluminescence mechanisms in anatase and rutile TiO2. J. Phys. Chem. C 2017, 121, 9011–9021. [Google Scholar] [CrossRef]
- Bergman, L.; McHale, J.L. Handbook of Luminescent Semiconductor Materials; CRC Press: Boca Raton, FL, USA, 2011; Volume 7. [Google Scholar]
- Lei, Y.; Zhang, L.D.; Meng, G.W.; Li, G.H.; Zhang, X.Y.; Liang, C.H.; Chen, W.; Wang, S.X. Preparation and photoluminescence of highly ordered TiO2 nanowire arrays. Appl. Phys. Lett. 2011, 78, 8. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Pavlenko, M.; Viter, R.; Jancelewicz, M.; Nowaczyk, G.; Baleviciute, I.; Załęski, K.; Jurga, S.; Ramanavicius, A.; Smyntyna, V. Tailoring the structural, optical, and photoluminescence properties of porous silicon/TiO2 nanostructures. J. Phys. Chem. C 2015, 119, 7164–7171. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Reduced and n-Type Doped TiO2: Nature of Ti3+ Species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]
- Gerosa, M.; Bottani, C.E.; Caramella, L.; Onida, G.; Di Valentin, C.; Pacchioni, G. Defect calculations in semiconductors through a dielectric-dependent hybrid DFT functional: The case of oxygen vacancies in metal oxides. J. Chem. Phys. 2015, 143, 134702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deak, P.; Aradi, B.; Frauenheim, T. Quantitative theory of the oxygen vacancy and carrier self-trapping in bulk TiO2. Phys. Rev. B 2012, 86, 195206. [Google Scholar] [CrossRef]
- Zhang, W.F.; Zhang, M.S.; Yin, Z.; Chen, Q. Photoluminescence in anatase titanium dioxide nanocrystals. Appl. Phys. B 2000, 70, 261–265. [Google Scholar] [CrossRef]
- Klotz, D.; Grave, D.A.; Rothschild, A. Accurate determination of the charge transfer efficiency of photoanodes for solar water-splitting. Phys. Chem. Chem. Phys. 2017, 19, 20383–20392. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.E.; Jang, J.W.; Liu, E.Y.; Wang, D. Understanding the origin of photoelectrode performance enhancement by probing surface kinetics. Chem. Sci. 2016, 7, 3347–3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachaus, C.; Abdi, F.F.; Peter, L.M.; Krol, R.V. Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci. 2017, 8, 3712–3719. [Google Scholar] [CrossRef] [PubMed]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water Oxidation at Hematite Photoelectrodes: The role of surface states. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.P.; Ghicov, A.; Schmuki, P. A photo-electrochemical investigation of self-organized TiO2 nanotubes. J. Electrochem. Soc. 2010, 157, 76–84. [Google Scholar] [CrossRef]
- Gurudayal; Chee, P.M.; Boix, P.P.; Ge, H.; Yanan, F.; Barber, J.; Wong, L.H. Core-shell hematite nanorods: A Simple method to improve the charge transfer in the photoanode for photoelectrochemical water-splitting. ACS Appl. Mater. Interfaces 2015, 7, 6852–6859. [Google Scholar] [CrossRef] [PubMed]
- Rex, R.E.; Knorr, F.J.; McHale, J.L. Surface Traps of TiO2 nanosheets and nanoparticles as illuminated by spectroelectrochemical photoluminescence. J. Phys. Chem. C 2014, 118, 16831–16841. [Google Scholar] [CrossRef]
- Tighineanu, A.; Ruff, T.; Albu, S.; Hahn, R.; Schmuki, P. Conductivity of TiO2 nanotubes: Influence of annealing time and temperature. Chem. Phys. Lett. 2010, 494, 260–263. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalavarthi, R.; Naldoni, A.; Kment, Š.; Mascaretti, L.; Kmentová, H.; Tomanec, O.; Schmuki, P.; Zbořil, R. Radiative and Non-Radiative Recombination Pathways in Mixed-Phase TiO2 Nanotubes for PEC Water-Splitting. Catalysts 2019, 9, 204. https://doi.org/10.3390/catal9020204
Yalavarthi R, Naldoni A, Kment Š, Mascaretti L, Kmentová H, Tomanec O, Schmuki P, Zbořil R. Radiative and Non-Radiative Recombination Pathways in Mixed-Phase TiO2 Nanotubes for PEC Water-Splitting. Catalysts. 2019; 9(2):204. https://doi.org/10.3390/catal9020204
Chicago/Turabian StyleYalavarthi, Rambabu, Alberto Naldoni, Štěpán Kment, Luca Mascaretti, Hana Kmentová, Ondřej Tomanec, Patrik Schmuki, and Radek Zbořil. 2019. "Radiative and Non-Radiative Recombination Pathways in Mixed-Phase TiO2 Nanotubes for PEC Water-Splitting" Catalysts 9, no. 2: 204. https://doi.org/10.3390/catal9020204