Comparison between a Water-Based and a Solvent-Based Impregnation Method towards Dispersed CuO/SBA-15 Catalysts: Texture, Structure and Catalytic Performance in Automotive Exhaust Gas Abatement
Abstract
:1. Introduction
2. Results
2.1. Textural Properties
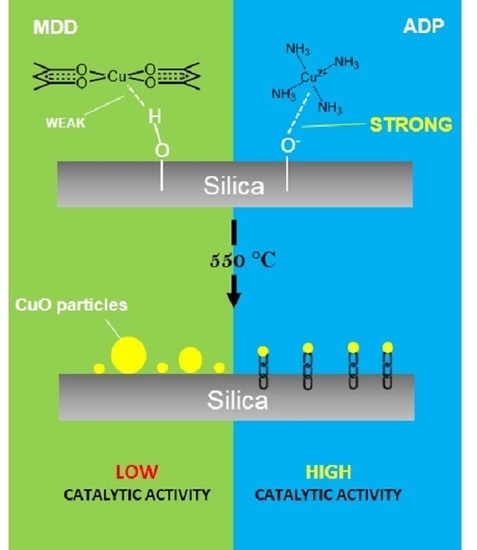
2.2. Copper Oxide Dispersion
2.3. The Formation of Copper Oxide
2.4. Catalytic Activity
3. Materials and Methods
3.1. Support Preparation
3.2. Catalysts Preparation
3.3. Catalysts Characterization
3.4. Catalytic Activity Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Twigg, M.V. Catalytic control of emissions from cars. Catal. Today 2011, 163, 33–41. [Google Scholar] [CrossRef]
- Muroi, T. Role of precious metal catalysts. In Noble Metals; Su, Y.H., Ed.; InTech: Rijeka, Croatia, 2012; pp. 301–330. [Google Scholar]
- Matthey, J. Forecast of Platinum Supply & Demands in 2014. Available online: www.platinum.matthey. com/documents/new-item/pgm%20market%20reports/pgm%20market%20report%20november%202015. pdf&usg=AFQjCNFcHLV5hPhuFOyFS2G7l19nhZEWBA&sig2=ZjbjI1fYY11VzQXbAezbNg&cad=rja (accessed on 22 August 2016).
- Fechete, I.; Wang, Y.; Védrine, J.C. The past, present and future of heterogeneous catalysis. Catal. Today 2012, 189, 2–27. [Google Scholar] [CrossRef]
- Ertl, G.; Knozinger, H.; Schutl, F.; Weitkamp, J. Handbook of Heterogenous Catalysis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 2237–2331. [Google Scholar]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J. Cu/Al2O3 catalysts for soot oxidation: Copper loading effect. Appl. Catal. B 2008, 84, 651–658. [Google Scholar] [CrossRef]
- Kummer, J.T. Catalysts for automobile emission control. Prog. Energy Combust. Sci. 1980, 6, 177–199. [Google Scholar] [CrossRef]
- Vila, F.; López Granados, M.; Ojeda, M.; Fierro, J.L.G.; Mariscal, R. Glycerol hydrogenolysis to 1,2-propanediol with Cu/γ-Al2O3: Effect of the activation process. Catal. Today 2012, 187, 122–128. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Fan, W.; Wang, J.; Li, Y. Highly efficient and robust Cu/SiO2 catalyst prepared by the ammonia evaporation hydrothermal method for glycerol hydrogenolysis to 1,2-propanediol. Catal. Sci. Technol. 2015, 5, 1169–1180. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Kang, H.; Chen, J.; Xia, C. Characterization and catalytic properties of the CuO/SiO2 catalysts prepared by precipitation-gel method in the hydrogenolysis of glycerol to 1,2-propanediol: Effect of residual sodium. Appl. Catal. A 2009, 366, 288–298. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Qiao, M.; Yan, S.; Li, H.; Shen, W.; Xu, H.; Fan, K. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Li, F.; Lu, C.-S.; Li, X.-N. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol. Chinese Chem. Lett. 2014, 25, 1461–1465. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wang, S.R.; Zhu, L.J.; Ge, X.L.; Li, X.B.; Luo, Z.Y. The influence of copper particle dispersion in Cu/SiO2 catalysts on the hydrogenation synthesis of ethylene glycol. Catal. Letters 2010, 135, 275–281. [Google Scholar] [CrossRef]
- van den Berg, R.; Parmentier, T.E.; Elkjær, C.F.; Gommes, C.J.; Sehested, J.; Helveg, S.; de Jongh, P.E.; de Jong, K.P. Support functionalization to retard ostwald ripening in copper methanol synthesis catalysts. ACS Catal. 2015, 5, 4439–4448. [Google Scholar] [CrossRef]
- Kasatkin, I.; Kurr, P.; Kniep, B.; Trunschke, A.; Schlögl, R. Role of lattice strain and defects in copper particles on the activity of Cu/ZnO/Al2O3 catalysts for methanol synthesis. Angew. Chemie Int. Ed. 2007, 46, 7324–7327. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.J.; Wang, L.; Han, P.D.; Huang, W. Methanol synthesis by CO and CO2 hydrogenation on Cu/γ-Al2O3 surface in liquid paraffin solution. Appl. Surf. Sci. 2014, 290, 398–404. [Google Scholar] [CrossRef]
- Jos van Dillen, A.; Terorde, R.; Lensveld, D.J.; Geus, J.W.; de Jong, K.P. Synthesis of supported catalysts by impregnation and drying using aqueous chelated metal complexes. J. Catal. 2003, 216, 257–264. [Google Scholar] [CrossRef]
- Twigg, M.V.; Spencer, M.S. Deactivation of supported copper metal catalysts for hydrogenation reactions. Appl. Catal. A 2001, 212, 161–174. [Google Scholar] [CrossRef]
- Prieto, G.; Zečević, J.; Friedrich, H.; de Jong, K.P.; de Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2013, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.A.; Campbell, C.T. Ceria maintains smaller metal catalyst particles by strong metal–support bonding. Science 2010, 329, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Kenvin, J.C.; White, M.G.; Mitchell, M.B. Preparation and characterization of supported mononuclear metal complexes as model catalysts. Langmuir 1991, 7, 1198–1205. [Google Scholar] [CrossRef]
- Lindblad, M.; Peter, L.; Suntola, T. Preparation of Ni/A12O3 catalysts from vapor phase by atomic layer epitaxy. Catal. Letters 1994, 27, 323–336. [Google Scholar] [CrossRef]
- Kuśtrowski, P.; Chmielarz, L.; Dziembaj, R.; Cool, P.; Vansant, E.F. Modification of MCM-48-, SBA-15-, MCF-, and MSU-type mesoporous silicas with transition metal oxides using the molecular designed dispersion method. J. Phys. Chem. B 2005, 109, 11552–11528. [Google Scholar] [CrossRef] [PubMed]
- Segura, Y.; Cool, P.; Van Der Voort, P.; Mees, F.; Meynen, V.; Vansant, E.F. TiOx-VOx mixed oxides on SBA-15 support prepared by the designed dispersion of acetylacetonate complexes: Spectroscopic study of the reaction mechanisms. J. Phys. Chem. B 2004, 108, 3794–3800. [Google Scholar] [CrossRef]
- Guo, X.; Yin, A.; Dai, W.L.; Fan, K. One pot synthesis of ultra-high copper contented Cu/SBA-15 material as excellent catalyst in the hydrogenation of dimethyl oxalate to ethylene glycol. Catal. Letters 2009, 132, 22–27. [Google Scholar] [CrossRef]
- Jiao, L.; Regalbuto, J.R. The synthesis of highly dispersed noble and base metals on silica via strong electrostatic adsorption: I. Amorphous silica. J. Catal. 2008, 260, 329–341. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Louis, C. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
- Dziembaj, R.; Cool, P.; Vansant, E.F. Catalytic performance of various mesoporous silicas modified with copper or iron oxides introduced by different ways in the selective reduction of NO by ammonia. Appl. Catal. B 2006, 62, 369–380. [Google Scholar]
- García-Trenco, A.; Martínez, A. A simple and efficient approach to confine Cu/ZnO methanol synthesis catalysts in the ordered mesoporous SBA-15 silica. Catal. Today 2013, 215, 152–161. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Prieto, G.; Shakeri, M.; de Jong, K.P.; de Jongh, P.E. Quantitative relationship between support porosity and the stability of pore-confined metal nanoparticles studied on CuZnO/SiO2 methanol synthesis catalysts. ACS Nano 2014, 8, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Van Der Grift, C.J.G.; Elberse, P.A.; Mulder, A.; Geus, J.W. Preparation of silica-supported copper catalysts by means of deposition-precipitation. Appl. Catal. 1990, 59, 275–289. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Louis, C. Metal particle size in silica-supported copper catalysts. Influence of the conditions of preparation and of thermal pretreatments. J. Phys. Chem. B 2000, 104, 965–972. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Xue, J.; Zuo, J.; Chen, J.; Xia, C. Cu/SiO2 catalysts prepared by hom- and heterogeneous deposition–precipitation methods: Texture, structure, and catalytic performance in the hydrogenolysis of glycerol to 1,2-propanediol. Catal. Today 2012, 183, 42–51. [Google Scholar] [CrossRef]
- Zhang, B.; Hui, S.; Zhang, S.; Ji, Y.; Li, W.; Fang, D. Effect of copper loading on texture, structure and catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol. J. Nat. Gas Chem. 2012, 21, 563–570. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Lynch, J.; Bazin, D.; Rebours, B.; Zanier, N.; Moisson, B.; Chaumette, P. Reducibility of cobalt species in silica-supported Fischer–Tropsch catalysts. J. Catal. 1997, 168, 16–25. [Google Scholar] [CrossRef]
- Praliaud, H.; Mikhailenko, S.; Chajar, Z.; Primet, M. Surface and bulk properties of Cu–ZSM-5 and Cu/Al2O3 solids during redox treatments. Correlation with the selective reduction of nitric oxide by hydrocarbons. Appl. Catal. B 1998, 16, 359–374. [Google Scholar] [CrossRef]
- Trouillet, L.; Toupance, T.; Villain, F.; Louis, C. In situ characterization of the coordination sphere of Cu II complexes supported on silica during the preparation of Cu/SiO2 catalysts by cation exchange. Phys. Chem. Chem. Phys. 2000, 2, 2005–2014. [Google Scholar] [CrossRef]
- Munnik, P.; Wolters, M.; Gabrielsson, A.; Pollington, S.D.; Headdock, G.; Bitter, J.H.; de Jongh, P.E.; de Jong, K.P. Copper nitrate redispersion to arrive at highly active silica-supported copper catalysts. J. Phys. Chem. C 2011, 115, 14698–14706. [Google Scholar] [CrossRef]
- Ryu, S.K.; Lee, W.K.; Park, S.J. Thermal decomposition of hydrated copper nitrate [Cu(NO3)2·3H2O] on activated carbon fibers. Carbon Sci. 2004, 5, 180–185. [Google Scholar]
- De Dobbelaere, C.; Mullens, J.; Hardy, A.; Van Bael, M.K. Thermal decomposition and spectroscopic investigation of a new aqueous glycolato(-peroxo) Ti(IV) solution–gel precursor. Thermochim. Acta 2011, 520, 121–133. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Bartholomew, C.H. Evironmetnal catalysis: Mobile souces. In Fundamentals of Industrial Catalytic Processes; Wiley: New York, NY, USA, 2004; pp. 705–741. [Google Scholar]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified syntheses of mesoporous materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Smeulders, G.; Meynen, V.; Silvestre-Albero, A.; Houthoofd, K.; Mertens, M.; Silvestre-Albero, J.; Martens, J.A.; Cool, P. The impact of framework organic functional groups on the hydrophobicity and overall stability of mesoporous silica materials. Mater. Chem. Phys. 2012, 132, 1077–1088. [Google Scholar] [CrossRef]








| Sample | Porosity | Metal Composition | ||||
|---|---|---|---|---|---|---|
| SBET 1 (m2·g−1) | Vmeso 2 (cm3·g−1) | Vmicro 3 (cm3·g−1) | VTotal 4 (cm3·g−1) | dp 5 (nm) | Cu (wt %) | |
| SBA-15 | 910 | 1.35 | 0.061 | 1.41 | 6.2 | – |
| MDD 6 | 737 | 1.36 | 0.010 | 1.37 | 6.7 | 7.1 |
| ADP 7 | 537 | 1.30 | 0.011 | 1.31 | 9.7 | 9.8 |
| Catalyst | CO | C3H6 | C3H8 | CH4 | NO | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | CMax (%) | T50 (°C) | CMax (%) | T50 (°C) | CMax (%) | T50 (°C) | CMax (%) | T50 (°C) | CMax (%) | |
| MDD | 410 | 80 | 325 | 100 | – | 25 | – | 0.6 | – | 8.0 |
| ADP | 202 | 100 | 260 | 100 | 430 | 85 | – | 10.7 | – | 4.0 |
| TWC | 222 | 100 | 225 | 100 | 370 | 89 | – | 9.7 | – | 40 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Q.; Glisenti, A.; Philippopoulos, C.; Poulakis, E.; Mertens, M.; Nyalosaso, J.L.; Meynen, V.; Cool, P. Comparison between a Water-Based and a Solvent-Based Impregnation Method towards Dispersed CuO/SBA-15 Catalysts: Texture, Structure and Catalytic Performance in Automotive Exhaust Gas Abatement. Catalysts 2016, 6, 164. https://doi.org/10.3390/catal6100164
Xin Q, Glisenti A, Philippopoulos C, Poulakis E, Mertens M, Nyalosaso JL, Meynen V, Cool P. Comparison between a Water-Based and a Solvent-Based Impregnation Method towards Dispersed CuO/SBA-15 Catalysts: Texture, Structure and Catalytic Performance in Automotive Exhaust Gas Abatement. Catalysts. 2016; 6(10):164. https://doi.org/10.3390/catal6100164
Chicago/Turabian StyleXin, Qi, Antonella Glisenti, Constantine Philippopoulos, Evangelos Poulakis, Myrjam Mertens, Jeff L. Nyalosaso, Vera Meynen, and Pegie Cool. 2016. "Comparison between a Water-Based and a Solvent-Based Impregnation Method towards Dispersed CuO/SBA-15 Catalysts: Texture, Structure and Catalytic Performance in Automotive Exhaust Gas Abatement" Catalysts 6, no. 10: 164. https://doi.org/10.3390/catal6100164