Experimental and Theoretical Studies of Sonically Prepared Cu–Y, Cu–USY and Cu–ZSM-5 Catalysts for SCR deNOx
Abstract
:1. Introduction
2. Results
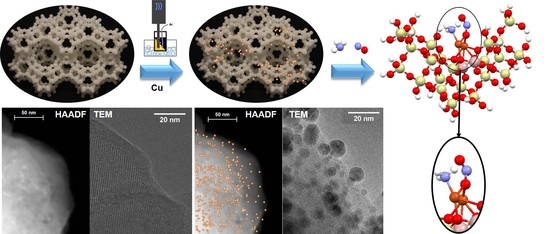
2.1. Catalysts Preparation and Characterization
2.2. Activity Measurements
2.3. In Situ DRIFT Studies
2.4. Theoretical Modeling
3. Discussion
3.1. Catalyst Characterization
3.2. Activity Measurements
3.3. In Situ DRIFT Studies
3.4. Theoretical Results and Reaction Mechanism
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.2.1. NO Evolution on Dehydrated Catalyst at Different Temperatures
4.2.2. NH3 Evolution on the Dehydrated Catalyst at Different Temperatures
4.2.3. SCR deNOx
4.3. Theoretical Modeling
5. Conclusions
- For Y/USY-type zeolites with a relatively low concentration of Brønsted acid sites (in the 21–70 μmol/g range), those sites are essential for deNOx reaction. NO reacts with hydrated copper, thereby releasing a water molecule with simultaneous NO adsorption. Subsequently, water is desorbed, and formation of the –N–NH complex between the bridge oxygen and the copper center is observed. In the final stage, N2 is formed with concurrent restoration of Brønsted acid sites of the copper dimer. In case of Y/USY, this process appears almost without barrier. For ZSM-5, the barrier is larger.
- For zeolites with a ZSM-5-type structure, a more energetically favorable process starts on the partly hydrated Cu–O–Cu dimer; during first step, two NO2 molecules react with the copper dimer, releasing nitric acid, and NO is adsorbed on the bridge oxygen. Subsequently, ammonium adsorbs on free copper centers. Subsequently, water is desorbed, forming the –N–NH complex on the bridge oxygen. In the last step, N2 is formed with concurrent restoration of the single hydroxyl group over the copper dimer. A similar path for Y/USY costs two barriers.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shelef, M. Selective Catalytic Reduction of NOx with N-Free Reductants. Chem. Rev. 1995, 95, 209–225. [Google Scholar] [CrossRef]
- Formation and control of nitrogen oxides. Catal. Today 1988, 2, 369–379. [CrossRef]
- Baran, R.; Grzybek, T.; Onfroy, T.; Dzwigaj, S. High activity of mononuclear copper present in the framework of CuSiBEA zeolites in the selective catalytic reduction of NO with NH3. Microporous Mesoporous Mater. 2016, 226, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Jodłowski, P.; Kryca, J.; Rogulska, A.; Gil, B.; Iwaniszyn, M.; Łojewska, J.; Kołodziej, A. Advantages of a wire gauze structured reactor with a zeolite (Cu-USY) catalyst for NH3-SCR of NOx. Chem. Eng. J. 2013, 214, 319–326. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Z.; Chen, Y.; Bao, W.; Chang, L.; Feng, G. In-situ hydrothermal synthesis of Cu-SSZ-13/cordierite for the catalytic removal of NOx from diesel vehicles by NH3. Chem. Eng. J. 2015, 263, 9–19. [Google Scholar] [CrossRef]
- Seo, C.-K.; Choi, B.; Kim, H.; Lee, C.-H. Effect of ZrO2 addition on de-NOx performance of Cu-ZSM-5 for SCR catalyst. Chem. Eng. J. 2012, 191, 331–340. [Google Scholar] [CrossRef]
- Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe modified derivatives of 2D MWW-type zeolites (MCM-22, ITQ-2 and MCM-36) as new catalysts for DeNOx process. Appl. Catal. B Environ. 2015, 168–169, 531–539. [Google Scholar] [CrossRef]
- Ayo, B.P.; De La Torre, U.; Sáez, M.R.; Aranzabal, A.; González-Marcos, J.A.; González-Velasco, J.R. Influence of the washcoat characteristics on NH3-SCR behavior of Cu-zeolite monoliths. Catal. Today 2013, 216, 82–89. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Riisager, A.; Fehrmann, R. Alkali resistant Cu/zeolite deNOx catalysts for flue gas cleaning in biomass fired applications. Appl. Catal. B Environ. 2011, 101, 183–188. [Google Scholar] [CrossRef]
- Kang, W.; Choi, B.; Kim, H. Characteristics of the simultaneous removal of PM and NOx using CuNb-ZSM-5 coated on diesel particulate filter. J. Ind. Eng. Chem. 2013, 19, 1406–1412. [Google Scholar] [CrossRef]
- Schay, Z.; Guczi, L.; Beck, A.; Nagy, I.; Samuel, V.; Mirajkar, S.; Ramaswamy, A.; Pál-Borbély, G. DeNOx reactions on Cu-zeolites: Decomposition of NO, N2O and SCR of NO by C3H8 and CH4 on Cu-ZSM-5 and Cu-AlTS-1 catalysts. Catal. Today 2002, 75, 393–399. [Google Scholar] [CrossRef]
- A Yashnik, S.; Ismagilov, Z. Cu-substituted ZSM-5 catalyst: Controlling of DeNO reactivity via ion-exchange mode with copper–ammonia solution. Appl. Catal. B Environ. 2015, 170–171, 241–254. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Guan, N.; Richter, M.; Fricke, R. Selective catalytic reduction of NO by propane in excess oxygen over IrCu-ZSM-5 catalyst. Catal. Commun. 2007, 8, 583–588. [Google Scholar] [CrossRef]
- Basaldella, E.; Kikot, A.; Quincoces, C.; González, M. Preparation of supported Cu/ZSM-5 zeolite films for DeNOx reaction. Mater. Lett. 2001, 51, 289–294. [Google Scholar] [CrossRef]
- De Lucas, A.; Valverde, J.; Dorado, F.; Romero, A.; Asencio, I. Influence of the ion exchanged metal (Cu, Co, Ni and Mn) on the selective catalytic reduction of NOX over mordenite and ZSM-5. J. Mol. Catal. A Chem. 2005, 225, 47–58. [Google Scholar] [CrossRef]
- Mrad, R.; Aissat, A.; Cousin, R.; Courcot, D.; Siffert, S. Catalysts for NOx selective catalytic reduction by hydrocarbons (HC-SCR). Appl. Catal. A Gen. 2015, 504, 542–548. [Google Scholar] [CrossRef]
- Heard, C.; Čejka, J.; Opanasenko, M.; Nachtigall, P.; Centi, G.; Perathoner, S. 2D Oxide Nanomaterials to Address the Energy Transition and Catalysis. Adv. Mater. 2018, 31, e1801712. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Yahiro, H. Novel catalytic decomposition and reduction of NO. Catal. Today 1994, 22, 5–18. [Google Scholar] [CrossRef]
- Halasz, I.; Brenner, A.; Ng, K.S.; Hou, Y. Catalytic Activity and Selectivity of H-ZSM5 for the Reduction of Nitric Oxide by Propane in the Presence of Oxygen. J. Catal. 1996, 161, 359–372. [Google Scholar] [CrossRef]
- Davydov, A. Molecular Spectroscopy of Oxide Catalyst Surfaces. Mol. Spectrosc. Oxide Catal. Surf. 2003. [Google Scholar] [CrossRef]
- De La Torre, U.; Ayo, B.P.; González-Velasco, J.R. Cu-zeolite NH3-SCR catalysts for NOx removal in the combined NSR–SCR technology. Chem. Eng. J. 2012, 207–208, 10–17. [Google Scholar] [CrossRef]
- Chen, P.; Simböck, J.; Schönebaum, S.; Rauch, D.; Simons, T.; Palkovits, R.; Moos, R.; Simon, U. Monitoring NH3 storage and conversion in Cu-ZSM-5 and Cu-SAPO-34 catalysts for NH3-SCR by simultaneous impedance and DRIFT spectroscopy. Sens. Actuators B Chem. 2016, 236, 1075–1082. [Google Scholar] [CrossRef]
- Rutkowska, M.; Pacia, I.; Basąg, S.; Kowalczyk, A.; Piwowarska, Z.; Duda, M.; Tarach, K.A.; Góra-Marek, K.; Michalik, M.; Díaz, U.; et al. Catalytic performance of commercial Cu-ZSM-5 zeolite modified by desilication in NH3 -SCR and NH3-SCO processes. Microporous Mesoporous Mater. 2017, 246, 193–206. [Google Scholar] [CrossRef]
- Günter, T.; Pesek, J.; Schäfer, K.; Abai, A.B.; Casapu, M.; Deutschmann, O.; Grunwaldt, J.-D. Cu-SSZ-13 as pre-turbine NOx-removal-catalyst: Impact of pressure and catalyst poisons. Appl. Catal. B Environ. 2016, 198, 548–557. [Google Scholar] [CrossRef]
- De La Torre, U.; Pereda-Ayo, B.; Romero-Sáez, M.; Aranzabal, A.; Pilar González-Marcos, M.; González-Marcos, J.A.; Gonzalez-Velasco, J.R. Screening of Fe–Cu-Zeolites Prepared by Different Methodology for Application in NSR–SCR Combined DeNOx Systems. Top. Catal. 2013, 56, 215–221. [Google Scholar] [CrossRef]
- Jodlowski, P.; Chlebda, D.; Piwowarczyk, E.; Chrzan, M.; Jędrzejczyk, R.; Sitarz, M.; Węgrzynowicz, A.; Kołodziej, A.; Łojewska, J. In situ and operando spectroscopic studies of sonically aided catalysts for biogas exhaust abatement. J. Mol. Struct. 2016, 1126, 132–140. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Suslick, K.S. Applications of Ultrasound to the Synthesis of Nanostructured Materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Andaç, Özlem; Telli, Ş.M.; Tatlier, M.; Erdem-Şenatalar, A.; Tatlıer, M. Effects of ultrasound on the preparation of zeolite A coatings. Microporous Mesoporous Mater. 2006, 88, 72–76. [Google Scholar] [CrossRef]
- Wang, B.; Wu, J.; Yuan, Z.-Y.; Li, N.; Xiang, S. Synthesis of MCM-22 zeolite by an ultrasonic-assisted aging procedure. Ultrason. Sonochem. 2008, 15, 334–338. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. Effects of ultrasonic treatment on zeolite synthesized from coal fly ash. Ultrason. Sonochem. 2011, 18, 661–668. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Fiore, S. Ultrasonic waves induce rapid zeolite synthesis in a seawater solution. Ultrason. Sonochem. 2013, 20, 32–36. [Google Scholar] [CrossRef]
- Pal, P.; Das, J.K.; Das, N.; Bandyopadhyay, S. Synthesis of NaP zeolite at room temperature and short crystallization time by sonochemical method. Ultrason. Sonochem. 2013, 20, 314–321. [Google Scholar] [CrossRef]
- Jodłowski, P.J.; Kuterasiński, Ł.; Jędrzejczyk, R.J.; Chlebda, D.; Gancarczyk, A.; Basąg, S.; Chmielarz, L. DeNOx Abatement Modelling over Sonically Prepared Copper USY and ZSM5 Structured Catalysts. Catalysis 2017, 7, 205. [Google Scholar] [CrossRef]
- Czekaj, I.; Brandenberger, S.; Kröcher, O. Theoretical studies of HNCO adsorption at stabilized iron complexes in the ZSM-5 framework. Microporous Mesoporous Mater. 2013, 169, 97–102. [Google Scholar] [CrossRef]
- Pulido, A.; Nachtigall, P. Correlation Between Catalytic Activity and Metal Cation Coordination: NO Decomposition Over Cu/Zeolites. ChemCatChem 2009, 1, 449–453. [Google Scholar] [CrossRef]
- Pulido, A.; Nachtigall, P. Theoretical investigation of dinitrosyl complexes in Cu-zeolites as intermediates in deNOx process. Phys. Chem. Chem. Phys. 2009, 11, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Jacobs, P.A.; Flanigen, E.M.; Jansen, J.C.; van Bekkum, H. Introduction to Zeolite Science and Practice; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Kuśtrowski, P.; Chmielarz, L.; Dziembaj, R.; Cool, A.P.; Vansant, E.F. Dehydrogenation of Ethylbenzene with Nitrous Oxide in the Presence of Mesoporous Silica Materials Modified with Transition Metal Oxides. J. Phys. Chem. A 2005, 109, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.M.; Schmal, M. The cyclohexanol dehydrogenation on RhCuAl2O3 catalysts Part 1. Characterization of the catalyst. Appl. Catal. A Gen. 1997, 151, 393–408. [Google Scholar] [CrossRef]
- Singh, L.; Rekha, P.; Chand, S. Cu-impregnated zeolite Y as highly active and stable heterogeneous Fenton-like catalyst for degradation of Congo red dye. Sep. Purif. Technol. 2016, 170, 321–336. [Google Scholar] [CrossRef]
- Macina, D.; Piwowarska, Z.; Tarach, K.A.; Góra-Marek, K.; Ryczkowski, J.; Chmielarz, L. Mesoporous silica materials modified with alumina polycations as catalysts for the synthesis of dimethyl ether from methanol. Mater. Res. Bull. 2016, 74, 425–435. [Google Scholar] [CrossRef]
- Rutkowska, M.; Piwowarska, Z.; Micek, E.; Chmielarz, L. Hierarchical Fe-, Cu- and Co-Beta zeolites obtained by mesotemplate-free method. Part I: Synthesis and catalytic activity in N2O decomposition. Microporous Mesoporous Mater. 2015, 209, 54–65. [Google Scholar] [CrossRef]
- Martins, L.; Peguin, R.; Wallau, M.; Urquieta, G. Cu-, Co-, Cu/Ca- and Co/Ca-exchanged ZSM-5 zeolites: Activity in the reduction of NO with methane or propane. Charact. Porous Solids III 2004, 154, 2475–2483. [Google Scholar] [CrossRef]
- Debbichi, L.; Lucas, M.D.C.M.D.; Pierson, J.-F.; Krueger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Auerbach, S.; Carrado, K.; Dutta, P. Microporous Materials Characterized by Vibrational Spectroscopies. Handb. Zeolite Sci. Technol. 2003. [Google Scholar] [CrossRef]
- LaValley, J. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catal. Today 1996, 27, 377–401. [Google Scholar] [CrossRef]
- Boroń, P.; Chmielarz, L.; Gil, B.; Marszałek, B.; Dzwigaj, S. Experimental evidence of NO SCR mechanism in the presence of the BEA zeolite with framework and extra-framework cobalt species. Appl. Catal. B Environ. 2016, 198, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Kryca, J.; Jodlowski, P.; Iwaniszyn, M.; Gil, B.; Sitarz, M.; Kołodziej, A.; Łojewska, T. Cu SSZ-13 zeolite catalyst on metallic foam support for SCR of NO with ammonia: Catalyst layering and characterisation of active sites. Catal. Today 2016, 268, 142–149. [Google Scholar] [CrossRef]
- Asmis, K.R.; Yang, Y.; Santambrogio, G.; Brummer, M.; Roscioli, J.R.; McCunn, L.R.; Johnson, M.A.; Kühn, O. Gas-Phase Infrared Spectroscopy and Multidimensional Quantum Calculations of the Protonated Ammonia Dimer N2H7+. Angew. Chem. Int. Ed. 2007, 46, 8691–8694. [Google Scholar] [CrossRef] [PubMed]
- Ochońska, J.; McClymont, D.; Jodlowski, P.; Knapik, A.; Gil, B.; Makowski, W.; Łasocha, W.; Kołodziej, A.; Kolaczkowski, S.; Łojewska, J. Copper exchanged ultrastable zeolite Y–A catalyst for NH3-SCR of NOx from stationary biogas engines. Catal. Today 2012, 191, 6–11. [Google Scholar] [CrossRef]
- Datka, J.; Kozyra, P. TPD–IR studies of CO desorption from zeolites CuY and CuX. J. Mol. Struct. 2005, 744–747, 991–996. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Gil, B.; Śliwa, M.; Datka, J. An IR spectroscopy study of Co sites in zeolites CoZSM-5. Appl. Catal. A Gen. 2007, 330, 33–42. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Gil, B.; Datka, J. Quantitative IR studies of the concentration of Co2+ and Co3+ sites in zeolites CoZSM-5 and CoFER. Appl. Catal. A Gen. 2009, 353, 117–122. [Google Scholar] [CrossRef]
- Tortorelli, M.; Chakarova, K.; Lisi, L.; Hadjiivanov, K. Disproportionation of associated Cu2+ sites in Cu-ZSM-5 to Cu+ and Cu3+ and FTIR detection of Cu3+(NO)x (x=1, 2) species. J. Catal. 2014, 309, 376–385. [Google Scholar] [CrossRef]
- Rejmak, P.; Broclawik, E.; Góra-Marek, K.; Radoń, M.; Datka, J. Nitrogen Monoxide Interaction with Cu(I) Sites in Zeolites X and Y: Quantum Chemical Calculations and IR Studies. J. Phys. Chem. C 2008, 112, 17998–18010. [Google Scholar] [CrossRef]
- Cheung, T.; Bhargava, S.K.; Hobday, M.; Foger, K. Adsorption of NO on Cu Exchanged Zeolites, an FTIR Study: Effects of Cu Levels, NO Pressure, and Catalyst Pretreatment. J. Catal. 1996, 158, 301–310. [Google Scholar] [CrossRef]
- Ochońska, J.; Regulska, A.; Jodlowski, P.; Iwaniszyn, M.; Michalik, M.; Łasocha, W.; Kołodziej, A.; Łojewska, J. Prospective catalytic structured converters for NH3-SCR of NOx from biogas stationary engines: In situ template-free synthesis of ZSM-5 Cu exchanged catalysts on steel carriers. Top. Catal. 2013, 56, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Kuterasiński, Ł.; Bodzioch, P.; Dymek, K.; Jędrzejczyk, R.; Chlebda, D.; Łojewska, J.; Sitarz, M.; Kurowski, G.; Jeleń, P.; Jodłowski, P. Spectroscopic studies of MFI and USY zeolite layers over stainless steel 316L wire gauze meshes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118060. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Liang, J.; Peng, H.; Yan, R.; Liu, W.; Wang, Z.; Wu, P.; Wang, X. Design and Synthesis of Cu/ZSM-5 Catalyst via a Facile One-Pot Dual-Template Strategy with Controllable Cu Content for Removal of NOx. Ind. Eng. Chem. Res. 2018, 57, 14967–14976. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Kamasamudram, K.; Epling, W.S. In Situ-DRIFTS Study of Selective Catalytic Reduction of NOx by NH3 over Cu-Exchanged SAPO-34. ACS Catal. 2013, 3, 871–881. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of Neutral and Charged NxOySurface Species by IR Spectroscopy. Catal. Rev. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Adelman, B.; Beutel, T.; Lei, G.-D.; Sachtler, W. Mechanistic Cause of Hydrocarbon Specificity over Cu/ZSM-5 and Co/ZSM-5 Catalysts in the Selective Catalytic Reduction of NOx. J. Catal. 1996, 158, 327–335. [Google Scholar] [CrossRef]
- Long, R.; Yang, R. Reaction Mechanism of Selective Catalytic Reduction of NO with NH3 over Fe-ZSM-5 Catalyst. J. Catal. 2002, 207, 224–231. [Google Scholar] [CrossRef]
- Bordiga, S.; Lamberti, C.; Bonino, F.; Travert, A.; Thibault-Starzyk, F. Probing zeolites by vibrational spectroscopies. Chem. Soc. Rev. 2015, 44, 7262–7341. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, A.G.; Marberger, A.; Thetford, A.; Lezcano-González, I.; Agote-Arán, M.; Nachtegaal, M.; Ferri, D.; Kröcher, O.; Catlow, C.R.A.; Beale, A.M. Detection of key transient Cu intermediates in SSZ-13 during NH3-SCR deNOx by modulation excitation IR spectroscopy. Chem. Sci. 2019, 11, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lezcano-Gonzalez, I.; Deka, U.; Arstad, B.; Deyne, A.V.Y.-D.; Hemelsoet, K.; Waroquier, M.; Van Speybroeck, V.; Weckhuysen, B.M.; Beale, A.M. Determining the storage, availability and reactivity of NH3 within Cu-Chabazite-based Ammonia Selective Catalytic Reduction systems. Phys. Chem. Chem. Phys. 2013, 16, 1639–1650. [Google Scholar] [CrossRef]
- Kuterasiński, Ł.; Filek, U.; Gackowski, M.; Zimowska, M.; Ruggiero-Mikołajczyk, M.; Jodłowski, P.J. Sonochemically prepared hierarchical MFI-type zeolites as active catalysts for catalytic ethanol dehydration. Ultras. Sonochem. 2021, 74, 105581. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, J.; Shen, M.; Li, W. NH3-SCR over Cu/SAPO-34 catalysts with various acid contents and low Cu loading. Catal. Sci. Technol. 2013, 3, 3234. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M. Selective catalytic reduction of NO over commercial DeNOx-catalysts: Experimental determination of kinetic and thermodynamic parameters. Chem. Eng. Sci. 1998, 53, 657–669. [Google Scholar] [CrossRef]
- Rivallan, M.; Ricchiardi, G.; Bordiga, S.; Zecchina, A. Adsorption and reactivity of nitrogen oxides (NO2, NO, N2O) on Fe–zeolites. J. Catal. 2009, 264, 104–116. [Google Scholar] [CrossRef]
- Forzatti, P.; Nova, I.; Tronconi, E. Enhanced NH3 Selective Catalytic Reduction for NOx Abatement. Angew. Chem. Int. Ed. 2009, 48, 8366–8368. [Google Scholar] [CrossRef] [PubMed]
- Deka, U.; Lezcano-Gonzalez, I.; Weckhuysen, B.M.; Beale, A.M. Local environment and nature of Cu active sites in zeolite-based catalysts for the selective catalytic reduction of NOx. ACS Catal. 2013, 3, 413–427. [Google Scholar] [CrossRef]
- Bendrich, M.; Scheuer, A.; Hayes, R.; Votsmeier, M. Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B Environ. 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Hermann, K.; Pettersson, L.G.M.; Casida, M.E.; Daul, C.; Goursot, A.; Koester, A.; Proynov, E.; St-Amant, A.; Salahub, D.R. StoBe-deMon Version 3.3 (2014). Available online: http://www.fhi-berlin.mpg.de/KHsoftware/StoBe/StoBeMAN.html (accessed on 6 July 2021).
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Labanowski, J.K.; Andzelm, J.W. Density Functional Methods in Chemistry; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Broclawik, E.; Salahub, D.R. Density functional theory and quantum chemistry: Metals and metal oxides. J. Mol. Catal. 1993, 82, 117–129. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Mayer, I. Bond orders and valences in the SCF theory: A comment. Theor. Chem. Acc. 1985, 67, 315–322. [Google Scholar] [CrossRef]
- Mayer, I. Bond orders and valences: Role of d-orbitals for hypervalent sulphur. J. Mol. Struct. 1987, 149, 81–89. [Google Scholar] [CrossRef]








| Catalyst | Preparation Method | Si/Al | Copper Content, wt% | SBET, m2/g | Vp Total, cm3/g | Acid Sites Conc. (NH3 sorption), μmol/gcat. | Active Cu Lewis Sites Conc. (CO Sorption), μmol/gcat. | |
|---|---|---|---|---|---|---|---|---|
| Brønsted | Lewis | |||||||
| Cu/Y | Ion exchange | 4.52 | 6.50 | 281.0 | 0.21 | 49 | 2116 | 72 |
| Cu/Y/s | Sonication | 4.52 | 3.82 | 137.4 | 0.12 | 70 | 2153 | 64 |
| Cu/USY | Ion exchange | 4.52 | 4.91 | 439.6 | 0.34 | 21 | 1452 | 184 |
| Cu/USY/s | Sonication | 4.52 | 3.72 | 450.8 | 0.34 | 63 | 1518 | 90 |
| Cu/ZSM-5-15 | Ion exchange | 15 | 0.62 | 283.1 | 0.26 | 229 | 355 | 196 |
| Cu/ZSM-5-15/s | Sonication | 15 | 0.10 | 317.0 | 0.28 | 272 | 365 | 60 |
| Cu/ZSM-5-37 | Ion exchange | 37 | 0.32 | 368.5 | 0.37 | 173 | 225 | 49 |
| Cu/ZSM-5-37/s | Sonication | 37 | 0.13 | 359.1 | 0.35 | 220 | 148 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jodłowski, P.J.; Czekaj, I.; Stachurska, P.; Kuterasiński, Ł.; Chmielarz, L.; Jędrzejczyk, R.J.; Jeleń, P.; Sitarz, M.; Górecka, S.; Mazur, M.; et al. Experimental and Theoretical Studies of Sonically Prepared Cu–Y, Cu–USY and Cu–ZSM-5 Catalysts for SCR deNOx. Catalysts 2021, 11, 824. https://doi.org/10.3390/catal11070824
Jodłowski PJ, Czekaj I, Stachurska P, Kuterasiński Ł, Chmielarz L, Jędrzejczyk RJ, Jeleń P, Sitarz M, Górecka S, Mazur M, et al. Experimental and Theoretical Studies of Sonically Prepared Cu–Y, Cu–USY and Cu–ZSM-5 Catalysts for SCR deNOx. Catalysts. 2021; 11(7):824. https://doi.org/10.3390/catal11070824
Chicago/Turabian StyleJodłowski, Przemysław J., Izabela Czekaj, Patrycja Stachurska, Łukasz Kuterasiński, Lucjan Chmielarz, Roman J. Jędrzejczyk, Piotr Jeleń, Maciej Sitarz, Sylwia Górecka, Michal Mazur, and et al. 2021. "Experimental and Theoretical Studies of Sonically Prepared Cu–Y, Cu–USY and Cu–ZSM-5 Catalysts for SCR deNOx" Catalysts 11, no. 7: 824. https://doi.org/10.3390/catal11070824