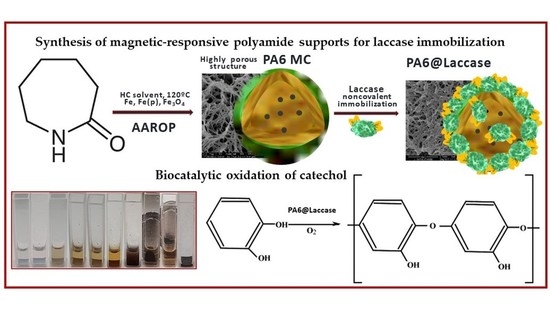
Magnetically Responsive PA6 Microparticles with Immobilized Laccase Show High Catalytic Efficiency in the Enzymatic Treatment of Catechol
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Morphology of the Empty Microparticulate Supports
2.2. Morphology and Laccase Content of the PA6-Laccase Conjugates
2.3. Structure Characterization of PA6 Empty Supports and Laccase Conjugates
2.3.1. FTIR Spectroscopy
2.3.2. Synchrotron WAXS
2.3.3. Synchrotron SAXS
2.3.4. Magnetization Studies
2.4. Enzymatic Activity of PA6@L Conjugates
2.5. Oxidation of Catechol by PA6@L Conjugates
2.6. Structure of Polycatechol Products
3. Materials and Methods
3.1. Materials
3.2. Instrumentation and Methods
3.3. Sample Preparation and Activity Testing
3.3.1. Synthesis of Empty or Magnetic PA6 Microparticulate Supports
3.3.2. Immobilization of Laccase by Physical Adsorption
3.3.3. Laccase Activity Assay
3.3.4. Determination of the Kinetics Parameters of the Enzymatic Oxidation
3.3.5. Kinetics of Enzymatic Oxidation of Catechol by Laccase-Immobilized PA6@L
3.3.6. Determination of the Total Content of Free OH Groups
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bull, A.T.; Bunch, A.W.; Robinson, G.K. Biocatalysts for clean industrial products and processes. Curr. Opin. Microbiol. 1999, 2, 246–251. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Van Rantwijk, F. Biocatalysis for sustainable organic synthesis. Aust. J. Chem. 2004, 57, 281–289. [Google Scholar] [CrossRef]
- Durán, N.; Rosa, M.A.; D’Annibale, A.; Liliana Gianfreda, L. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzym. Microb. Technol. 2002, 31, 907–931. [Google Scholar] [CrossRef]
- Ge, J.; Lu, D.; Liu, Z.; Liu, Z. Recent advances in nanostructured biocatalysts. Biochem. Eng. J. 2009, 44, 53–59. [Google Scholar] [CrossRef]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilization. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.; Magner, E.; Cooney, J.; Hodnett, B. Methodology for the immobilization of enzymes onto mesoporous materials. J. Phys. Chem. B 2005, 109, 19496–19506. [Google Scholar] [CrossRef] [PubMed]
- Torres-Salas, P.; Monte-Martinez, A.; Cutiño-Avila, B.; Rodriguez-Colinas, B.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Immobilized Biocatalysts—Novel approaches and tools for binding enzymes to supports. Adv. Mater. 2011, 23, 5275–5282. [Google Scholar] [CrossRef] [Green Version]
- Sigurdardóttir, S.B.; Lehmann, J.; Ovtar, S.; Grivel, J.C.; Negra, M.; Kaiser, A.; Pinelo, M. Enzyme immobilization on inorganic surfaces for membrane reactor applications: Mass transfer challenges, enzyme leakage and reuse of materials. Adv. Synth. Catal. 2018, 360, 2578–2607. [Google Scholar] [CrossRef]
- Shakya, A.K.; Nandakumar, K.S. An update on smart biocatalysts for industrial and biomedical applications. J. R. Soc. Interface 2018, 15, 20180062. [Google Scholar] [CrossRef]
- Gitsov, I.; Hamzik, J.; Ryan, J.; Simonyan, A.; Nakas, J.P.; Omori, S.; Krastanov, A.; Cohen, T.; Tanenbaum, S.W. Enzymatic nanoreactors for environmentally benign biotransformations—1. Formation and catalytic activity of supramolecular complexes of laccase and linear-dendritic block copolymers. Biomacromolecules 2008, 9, 804–811. [Google Scholar] [CrossRef]
- Scheibel, D.M.; Gitsov, I. Polymer-assisted biocatalysis: Effects of macromolecular architectures on the stability and catalytic activity of immobilized enzymes toward water-soluble and water-insoluble substrates. ACS Omega 2018, 3, 1700–1709. [Google Scholar] [CrossRef] [Green Version]
- Mate, D.M.; Alcalde, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef]
- Couto, S.L.; Herrera, J.L. Lacasses in the textile industry. Biotechnol. Mol. Biol. Rev. 2006, 1, 115–120. Available online: https://academicjournals.org/journal/BMBR/article-full-text-pdf/1C6FD3040214 (accessed on 20 October 2020).
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheibel, D.M.; Guo, D.; Luo, J.; Gitsov, I. A Single Enzyme Mediates the “Quasi-Living” Formation of Multiblock Copolymers with a Broad Biomedical Potential. Biomacromolecules 2020, 21, 2132–2146. [Google Scholar] [CrossRef] [PubMed]
- Gitsov, I.; Wang, L.; Vladimirov, N.; Simonyan, A.; Kiemle, D.J.; Schütz, A. “Green” synthesis of unnatural poly(amino acid)s with zwitterionic character and pH-responsive solution behavior, mediated by linear-dendritic laccase complexes. Biomacromolecules 2014, 15, 4082–4095. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, D.M.; Gitsov, I. Unprecedented enzymatic synthesis of perfectly structured alternating copolymers via “green” reaction cocatalyzed by laccase and lipase compartmentalized within supramolecular complexes. Biomacromolecules 2019, 20, 927–936. [Google Scholar] [CrossRef]
- Gitsov, I.; Simonyan, A. “Green” synthesis of bisphenol polymers and copolymers, mediated by supramolecular complexes of laccase and linear-dendritic block copolymers. In Green Polymer Chemistry: Biocatalysis and Materials II; Cheng, H.N., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2013; Volume 1144, pp. 121–139. [Google Scholar]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.N.; Zhao, Y. Hazardous contaminants in the environment and their laccase-assisted degradation—A review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Claus, H.; Faber, G.; König, H. Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl. Microbiol. Biotechnol. 2002, 59, 672–678. [Google Scholar] [CrossRef]
- Maloney, J.; Dong, C.; Campbell, A.S.; Dinu, C.Z. Emerging enzyme-based technologies for wastewater treatment. In Green Polymer Chemistry: Biobased Materials and Biocatalysis; American Chemical Society: Washington, DC, USA, 2015; Volume 1192, pp. 73–75. [Google Scholar]
- Cermola, F.; Dellagreca, M.; Iesce, M.R.; Montella, S.; Pollio, A.; Temussi, F. A mild photochemical approach to the degradation of phenols from olive oil mill wastewater. Chemosphere 2004, 55, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, N.; Zehnder, A.J.B.; Eggen, R.I.L. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, J.; Li, G.; Zhang, J.; Li, D.; Huang, F.; Wei, Q. Laccase immobilized on a PAN/adsorbents composite nanofibrous membrane for catechol treatment by a biocatalysis/adsorption process. Molecules 2014, 19, 3376–3388. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Noro, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Enzymatic polymerization of catechol under high-pressure homogenization for the green coloration of textile. J. Clean. Prod. 2008, 202, 792–798. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Noro, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Exploring PEGylated and immobilized laccases for catechol polymerization. AMB Expr. 2018, 8, 134. [Google Scholar] [CrossRef]
- Aktas, N.; Sahiner, N.; Kantoglu, Ö.; Salih, B.; Tanyolac, A. Biosynthesis and characterization of laccase catalyzed poly(catechol). J. Polym. Environ. 2003, 11, 123–128. [Google Scholar] [CrossRef]
- Aktas, N.; Tanyolac, A. Reaction conditions for laccase catalyzed polymerization of catechol. Biores. Technol. 2003, 87, 209–214. [Google Scholar] [CrossRef]
- Marczewska, B.; Przegalinski, M. Poly(catechol) electroactive film and its electrochemical properties. Synth. Met. 2013, 182, 33–39. [Google Scholar] [CrossRef]
- Gavrilas, S.; Dumitriu, F.; Stanescu, M.D. Commercial laccase oxidation of laccase compounds. U.P.B. Sci. Bull. Ser. B Chem Mater. Sci. 2012, 74, 3–10. [Google Scholar]
- Stanescu, M.D.; Gavrilas, S.; Ludwig, R.; Haltrich, D.; Lozinsky, V.I. Preparation of immobilized Trametes pubescens laccase on a cryogel-type polymeric carrier and application of the biocatalyst to apple juice phenolic compounds oxidation. Eur. Food Res. Technol. 2012, 234, 655–662. [Google Scholar] [CrossRef]
- Piacquadio, P.; De Stefano, G.; Sammartino, M.; Sciancalepore, V. Phenols removal from apple juice by laccase immobilized on Cu2+-chelate regenerable carrier. Biotechnol. Tech. 1997, 11, 515–517. [Google Scholar] [CrossRef]
- Hadzhiyska, H.; Calafell, M.; Gibert, J.M.; Daga, J.M.; Tzanov, T. Laccase-assisted dyeing of cotton. Biotechnol. Let. 2006, 28, 755–759. [Google Scholar] [CrossRef]
- Kim, S.; Silva, C.; Evtuguin, D.V.; Gamelas, J.A.F.; Cavaco-Paulo, A. Polyoxometalate/laccase-mediated oxidative polymerization of catechol for textile dyeing. Appl. Microbiol. Biotechnol. 2011, 89, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.; Guebitz, G.; Cavaco-Paulo, A. In situ enzymatically prepared polymers for wool coloration. Macromol. Mater. Eng. 2001, 286, 691–694. [Google Scholar] [CrossRef]
- Fatarella, E.; Spinelli, D.; Ruzzante, M.; Pogni, R. Nylon 6 film and nanofiber carriers: Preparation and laccase immobilization performance. J. Mol. Catal. B Enzym. 2014, 102, 41–47. [Google Scholar] [CrossRef]
- Jasni, M.J.F.; Sathishkumar, P.; Sornambikai, S.; Yusoff, A.R.M.; Ameen, F.; Buang, N.A.; Kadir, M.R.A.; Yusop, Z. Fabrication, characterization and application of laccase–nylon 6,6/Fe3+ composite nanofibrous membrane for 3,30-dimethoxybenzidine detoxification. Bioprocess Biosyst. Eng. 2017, 40, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Silva, C.J.; Zille, A.; Guebitz, G.M.; Cavaco-Paulo, A. Laccase immobilization on enzymatically functionalized polyamide 6,6 fibers. Enzym. Microb. Technol. 2007, 41, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Dencheva, N.; Denchev, Z.; Lanceros-Méndez, S.; Ezquerra, T.A. One-step in-situ synthesis of polyamide microcapsules with inorganic payload and their transformation into responsive thermoplastic composite materials. Macromol. Mater. Eng. 2016, 301, 119–124. [Google Scholar] [CrossRef]
- Cano-Raya, C.C.; Dencheva, N.V.; Braz, J.F.; Malfois, M.; Denchev, Z. Optical biosensor for catechol determination based on laccase-immobilized anionic polyamide 6 microparticles. J. Appl. Polym. Sci. 2020, 137, 49131. [Google Scholar] [CrossRef]
- Dencheva, N.; Braz, J.; Nunes, T.G.; Oliveira, F.D.; Denchev, Z. One-pot low temperature synthesis and characterization of hybrid poly(2-pyrrolidone) microparticles suitable for protein immobilization. Polymer 2018, 145, 402–4015. [Google Scholar] [CrossRef]
- Dencheva, N.V.; Oliveira, F.D.; Braz, J.F.; Denchev, Z. Bovine serum albumin-imprinted magnetic poly(2-pyrrolidone) microparticles for protein recognition. Eur. Polym. J. 2020, 122, 109375. [Google Scholar] [CrossRef]
- Dencheva, N.; Braz, J.; Scheibel, D.; Malfois, M.; Denchev, Z.; Gitsov, I. Polymer-assisted biocatalysis: Polyamide 4 microparticles as promising carriers of enzymatic function. Catalysts 2020, 10, 767. [Google Scholar] [CrossRef]
- Oliveira, F.M.; Dencheva, N.V.; Lanceros-Méndez, S.; Nunes, T.G.; Denchev, Z. Binary Polyamide Hybrid Composites Containing Carbon Allotropes and Metal Particles with Radiofrequency Shielding Effect. Polym. Compos. 2019, 40, E1338–E1352. [Google Scholar] [CrossRef]
- Liers, C.; Ullrich, R.; Pecyna, M.; Schlosser, D.; Hofrichter, M. Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylaria polymorpha. Enzym. Microb. Technol. 2007, 41, 785–793. [Google Scholar] [CrossRef]
- Atalla, M.; Zeinab, H.; Eman, R.; Amani, A.; Abeer, A. Characterization and kinetic properties of the purified Trematosphaeria mangrovei laccase enzyme. Saudi J. Bioll. Sci. 2013, 20, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavares, A.P.M.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Qiu, H.; Xu, C.; Huang, X.; Ding, Y.; Qu, Y.; Gao, P. Immobilization of laccase on nanoporous gold: Comparative studies on the immobilization strategies and the particle size effects. J. Phys. Chem. C 2009, 113, 2521–2525. [Google Scholar] [CrossRef]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from Trametes versicolor at 1.90 Å resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dencheva, N.; Nunes, T.; Oliveira, M.J.; Denchev, Z. Microfibrillar composites based on polyamide/polyethylene blends. 1. Structure investigations in oriented and isotropic PA6. Polymer 2005, 46, 887–901. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Crystallization behavior of Nylon nanocomposites. Polymer 2003, 44, 3945–3961. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Argon, A.S.; Cohen, R.E. On the plastic deformation of the amorphous component in semicrystalline polymers. Polymer 1996, 37, 2113–2123. [Google Scholar] [CrossRef]
- Alexander, L.E. X-ray Diffraction Methods in Polymer Science; Wiley-Interscience: New York, NY, USA, 1969. [Google Scholar]
- Bolivar, J.M.; Consolati, T.; Mayr, T.; Nidetzky, B. Shine a light on immobilized enzymes: Real-time sensing in solid supported biocatalysts. Trends Biotechnol. 2013, 31, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Milagres, A.M.F. The synergistic action of ligninolytic enzymes (MnP and Laccase) and Fe3+-reducing activity from white-rot fungi for degradation of Azure B. Enzym. Microb. Technol. 2007, 42, 17–22. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, L.; Li, G.; Zhang, P.; Li, D.; Huang, F.; Wei, Q. Activity of laccase immobilized on TiO2-Montmorillonite complexes. Int. J. Mol. Sci. 2013, 14, 12520–12532. [Google Scholar] [CrossRef] [Green Version]
- Johannes, C.; Majcherczyk, A. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl. Environ. Microbiol. 2000, 66, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fang, X.; Yuan, J.; Li, C.; Wang, P. Laccase-catalyzed oxidative polymerization of phenolic compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef]
- Ito, S.; Sugumaran, M.; Kazumasa, W. Chemical reactivities of ortho-quinones produced in living organisms: Fate of quinonoid products formed by tyrosinase and phenoloxidase action on phenols and catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Su, J.; Castro, T.G.; Noro, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. The effect of high-energy environments on the structure of laccase polymerized poly(catechol). Ultrason. Sonochem. 2018, 48, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Song, J.E.; Su, J.; Noro, J.; Cavaco-Paulo, A.; Silva, C.; Kim, H.R. Bio-coloration of bacterial cellulose assisted by immobilized laccase. AMB Expr. 2018, 8, 19–30. [Google Scholar] [CrossRef] [Green Version]
- González, J.B.; González, N.; Colldelram, C.; Ribó, L.; Fontserè, A.; Manas, G.J.; Villanueva, J.; Llonch, M.; Peña, G.; Gevorgyan, A.; et al. NCD-SWEET beamline upgrade. In Proceedings of the 10th Mechanical Engineering Design of Synchrotron Radiation Equipment and Instrumentation Conference, Paris, France, 25–29 June 2018; pp. 374–376. [Google Scholar]
- Ashiotis, G.; Deschildre, A.; Nawaz, Z.; Wright, J.P.; Karkoulis, D.; Picca, F.E.; Kieffer, J. The fast azimuthal integration Python library: pyFAI. J. Appl. Crystallogr. 2015, 48, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Agbor, G.A.; Vinson, J.A.; Donnelly, P.A. Folin-Ciocalteau reagent for polyphenolic assay. Int. J. Food Sci. Nutr. Diet. 2014, 3, 147–156. [Google Scholar] [CrossRef]













| Sample | PA6 Yield, % a | % of Oligo-mers | Real Fe Content, RL, % b | [η], dL.g−1 | dmax, µm | dmax/dmin |
|---|---|---|---|---|---|---|
| PA6 | 47.8 | 2.4 | - | 0.983 | 30–35 | 1.2–1.4 |
| PA6Fe | 50.2 | 3.3 | 6.29 | 30–35 | 1.2–1.3 2.1–2.5 | |
| PA6FeP | 45.4 | 2.7 | 6.25 | 30–35 55–60 | 1.1–2.2 | |
| PA6Fe3O4 | 41.5 | 2.3 | 6.05 | 35–40 | 1.2–1.3 |
| Sample | Residual Laccase [mg/mL] a | Laccase on Support, [mg] b | Laccase per g Support [mg/g] | IE,c [%] | µm | |
|---|---|---|---|---|---|---|
| PA6-L | 0.17 | 14.6 | 29.3 | 91.5 | 3–6 | 1.2–1.6 |
| PA6Fe-L | 0.38 | 12.9 | 25.9 | 80.8 | 4–8 | 1.2–1.5 2.1–2.5 |
| PA6FeP-L | 0.19 | 14.5 | 29.0 | 90.6 | 4–6 | 1.2–1.5 2.1–2.5 |
| PA6Fe3O4-L | 0.45 | 12.4 | 24.7 | 77.3 | 4–8 | 1.2–1.6 |
| Sample | α—PA6 % | γ—PA6 % | α/γ | |||
|---|---|---|---|---|---|---|
| Laccase | - | - | - | - | 12.9 | 0.0 |
| PA6 | 32.7 | 11.6 | 44.3 | 2.82 | - | - |
| PA6-L | 20.7 | 17.6 | 44.1 | 1.18 | 19.5 | 6.7 |
| PA6Fe-L | 20.8 | 13.8 | 40.0 | 1.51 | 19.7 | 6.8 |
| PA6FeP-L | 19.9 | 14.3 | 40.0 | 1.39 | 20.0 | 7.1 |
| PA6Fe3O4-L | 20.1 | 18.1 | 43.2 | 1.11 | 19.5 | 6.6 |
| Sample | Laccase Activity [µkat L−1] | Specific Activity per mg Laccase | Specific Activity per mg lacc. and mg MP | Relative Laccase Activity, [%] | Km, mM | Vmax, [µmol L−1 s−1 mg lacc−1] |
|---|---|---|---|---|---|---|
| Free laccase | 2.67 | 22.23 | - | 100.0 | 0.27 | 5.48 |
| PA6-L | 2.37 | 18.13 | 3.63 | 81.5 | 0.70 | 5.99 |
| PA6Fe-L | 2.67 | 20.82 | 4.16 | 93.6 | 1.46 | 8.71 |
| PA6FeP-L | 3.39 | 23.49 | 4.70 | 105.7 | 4.31 | 9.74 |
| PA6Fe3O4-L | 2.60 | 21.25 | 4.25 | 95.6 | 2.42 | 6.03 |
| Oxidation Cycle | Sample | Enzymatic Catechol Oxidation Rate Constant, k, min−1 | Visual Representation of 5 Consecutive Catechol Oxidation Cycles |
|---|---|---|---|
| 5 | PA6-L | 0.0043 ± 2.69.10−4 |  |
| PA6Fe-L | 0.0042 ± 1.89.10−4 | ||
| PA6FeP-L | 0.0045 ± 1.32.10−4 | ||
| PA6Fe3O4-L | 0.0044 ± 2.01.10−4 | ||
| 4 | PA6-L | 0.0046 ± 7.88.10−5 |  |
| PA6Fe-L | 0.0050 ± 9.61.10−5 | ||
| PA6FeP-L | 0.0061 ± 3.50.10−4 | ||
| PA6Fe3O4-L | 0.0050 ± 9.66.10−5 | ||
| 3 | PA6-L | 0.0061 ± 7.31.10−5 |  |
| PA6Fe-L | 0.0051 ± 9.37.10−5 | ||
| PA6FeP-L | 0.0069 ± 2.35.10−4 | ||
| PA6Fe3O4-L | 0.0051 ± 1.58.10−4 | ||
| 2 | PA6-L | 0.0085 ± 3.81.10−4 |  |
| PA6Fe-L | 0.0082 ± 3.87.10−4 | ||
| PA6FeP-L | 0.0086 ± 2.16.10−4 | ||
| PA6Fe3O4-L | 0.0071 ± 1.23.10−4 | ||
| 1 | PA6-L | 0.0189 ± 5.44.10−4 |  |
| PA6Fe-L | 0.0193 ± 7.82.10−4 | ||
| PA6FeP-L | 0.0203 ± 7.57.10−4 | ||
| PA6Fe3O4-L | 0.0165 ± 1.58.10−4 | ||
| - | Free Laccase | 0.0194 ± 7.82.10−4 |
| Sample | Absorbance at 750 nm | Free OH, a.u. Gallic acid | Free OH µmol Catechol | Free OH Group Retention | |
|---|---|---|---|---|---|
| Catechol 20 mL 25 mM | 0.450 | 518.102 | 0.0250 | 100.0 | |
| Free laccase | 0.148 | 148.963 | 0.0072 | 28.8 | |
| I cycle | PA6-L | 0.242 | 263.861 | 0.0127 | 50.9 |
| PA6Fe-L | 0.254 | 278.529 | 0.0134 | 53.8 | |
| PA6FeP-L | 0.204 | 217.413 | 0.0105 | 42.0 | |
| PA6Fe3O4-L | 0.241 | 262.638 | 0.0127 | 50.7 | |
| II cycle | PA6-L | 0.291 | 323.754 | 0.0156 | 62.5 |
| PA6Fe-L | 0.335 | 377.536 | 0.0182 | 72.9 | |
| PA6FeP-L | 0.289 | 321.309 | 0.0155 | 62.0 | |
| PA6Fe3O4-L | 0.386 | 439.874 | 0.0212 | 84.9 | |
| III cycle | PA6-L | 0.358 | 405.649 | 0.0196 | 78.3 |
| PA6Fe-L | 0.369 | 419.095 | 0.0202 | 80.9 | |
| PA6FeP-L | 0.309 | 345.756 | 0.0167 | 66.7 | |
| PA6Fe3O4-L | 0.405 | 463.098 | 0.0223 | 89.4 | |
| IV cycle | PA6-L | 0.364 | 412.983 | 0.0199 | 79.7 |
| PA6Fe-L | 0.376 | 427.651 | 0.0206 | 82.5 | |
| PA6FeP-L | 0.316 | 354.312 | 0.0171 | 68.4 | |
| PA6Fe3O4-L | 0.378 | 430.095 | 0.0208 | 83.0 | |
| V cycle | PA6-L | 0.371 | 421.539 | 0.0203 | 81.4 |
| PA6Fe-L | 0.385 | 438.652 | 0.0212 | 84.7 | |
| PA6FeP-L | 0.324 | 364.090 | 0.0176 | 70.3 | |
| PA6Fe3O4-L | 0.396 | 452.097 | 0.0218 | 87.3 | |
| Solid residue after cycle V | Free laccase * | 0.085 | 71.958 | 0.0035 | 13.9 |
| PA6-L | 0.035 | 23.278 | 0.0011 | 4.5 | |
| PA6Fe-L | 0.023 | 9.027 | 0.0004 | 1.7 | |
| PA6FeP-L | 0.016 | 0.119 | 0.0001 | 0.0 | |
| PA6Fe3O4-L | 0.270 | 13.777 | 0.0007 | 2.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dencheva, N.; Oliveira, S.; Braz, J.; Getya, D.; Malfois, M.; Denchev, Z.; Gitsov, I. Magnetically Responsive PA6 Microparticles with Immobilized Laccase Show High Catalytic Efficiency in the Enzymatic Treatment of Catechol. Catalysts 2021, 11, 239. https://doi.org/10.3390/catal11020239
Dencheva N, Oliveira S, Braz J, Getya D, Malfois M, Denchev Z, Gitsov I. Magnetically Responsive PA6 Microparticles with Immobilized Laccase Show High Catalytic Efficiency in the Enzymatic Treatment of Catechol. Catalysts. 2021; 11(2):239. https://doi.org/10.3390/catal11020239
Chicago/Turabian StyleDencheva, Nadya, Sandra Oliveira, Joana Braz, Dariya Getya, Marc Malfois, Zlatan Denchev, and Ivan Gitsov. 2021. "Magnetically Responsive PA6 Microparticles with Immobilized Laccase Show High Catalytic Efficiency in the Enzymatic Treatment of Catechol" Catalysts 11, no. 2: 239. https://doi.org/10.3390/catal11020239