Comparison of the Photocatalytic Activity of ZnO/CeO2 and ZnO/Yb2O3 Mixed Systems in the Phenol Removal from Water: A Mechanicistic Approach
Abstract
:1. Introduction
2. Results
2.1. Structural Analysis
2.2. Optical Analysis
2.3. EPR Characterization
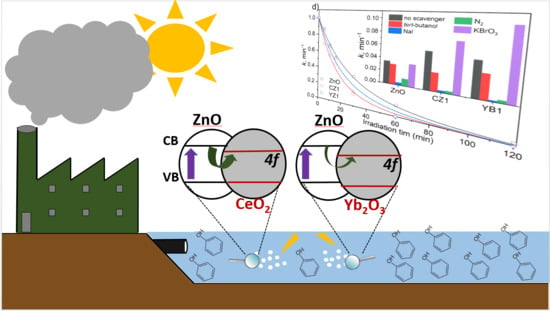
2.4. Photocatalytic Activity and Effect of Scavenger Addition
3. Discussion
4. Materials and Methods
4.1. Samples Preparation
4.2. Samples Characterization
4.3. Photocatalytic Degradation Experiments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewis, N.S. Toward Cost-Effective Solar Energy Use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Ani, I.J.; Akpan, U.G.; Olutoye, M.A.; Hameed, B.H. Photocatalytic Degradation of Pollutants in Petroleum Refinery Wastewater by TiO2- and ZnO-based Photocatalysts: Recent Development. J. Clean. Prod. 2018, 205, 930–954. [Google Scholar] [CrossRef]
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Destruction of Water Contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Bahnemann, D. Photocatalytic Water Treatment: Solar Energy Applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial Photosynthesis for Solar Water-Splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Domen, K.; Kondo, J.N.; Hara, M.; Takata, T. Photo- and Mechano-Catalytic Overall Water Splitting Reactions to form Hydrogen and Oxygen on Heterogeneus Catalyst. Bull. Chem. Soc. Jpn. 2000, 73, 1307–1331. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Vu, N.N.; Kaliaguine, S.; Do, T.O. Critical Aspects and Recent Advances in Structural Engineering of Photocatalysts for Sunlight-Driven Photocatalytic Reduction of CO2 into Fuels. Adv. Funct. Mater. 2019, 29, 1901825. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, A.; Medina-Ramirez, I. Photocatalytic Semiconductors; Springer International Publishing: Chem, Switzerland, 2015. [Google Scholar]
- Theerthagiri, J.; Chandrasekaran, S.; Salla, S.; Elakkiya, V.; Senthil, R.A.; Nithyadharseni, P.; Maiyalagan, T.; Micheal, K.; Ayeshamariam, A.; Arasu, M.V.; et al. Recent Developments of Metal Oxide Based Heterostructures for Photocatalytic Applications Towards Environmental Remediation. J. Solid State Chem. 2018, 267, 35–52. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous Photocatalyst Materials for Water Splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nat. Biotechnol. 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Sato, S. Photocatalytic Activity of NOX-doped TiO2 in the Visible Light Region. Chem. Phys. Lett. 1986, 123, 126–128. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Valentin, C.D.; Pacchioni, G. Origin of Photoactivity of Nitrogen-Doped Titanium Dioxide under Visible Light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent Advances in Photocatalysis for Environmental Applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Emeline, A.V.; Kuznetsov, V.N.; Ryabchuk, V.K.; Serpone, N. On the way to the creation of next generation photoactive materials. Environ. Sci. Pollut. Res. 2012, 19, 3666–3675. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis-Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A. Engineered Nanomaterials for Water Decontamination and Purification: From Lab to Products. J. Hazard. Mater. 2019, 363, 295–308. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and Multi Metal Oxide Nanoparticles: Synthesis, Antibacterial and Cytotoxic Properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Walle, C.G.V.d. Fundamentals of Zinc Oxide as a ASemiconductor. Rep. Prog. Phys. 2009, 72, 126501–1265028. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Litton, C.W.; Collins, T.C. Zeeman Effects in the Edge Emission and Absorption of ZnO. Phys. Rev. 1965, 140, A1726–A1734. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301–041301103. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Khodyuk, I.V. Optical and Luminescence Properties of Zinc Oxide (Review). Opt. Spectrosc. 2011, 111, 776–785. [Google Scholar] [CrossRef]
- Hoffmann, R.M.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Klingshirn, C.; Fallert, J.; Zhou, H.; Sartor, J.; Thiele, C.; Maier-Flaig, F.; Schneider, D.; Kalt, H. 65 Cears of ZnO Research-Old and Very Recent Results. Phys. Stat. Sol. B 2010, 247, 1424–1447. [Google Scholar] [CrossRef]
- Cerrato, E.; Paganini, M.C.; Giamello, E. Photoactivity under Visible Light of Defective ZnO Investigated by EPR Spectroscopy and Photoluminescence. J. Photochem. Photobiol., A 2020, 397, 112531. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent Progress on Doped ZnO Nanostructures for Visible-Light Photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Abebe, B.; Murthy, H.C.A.; Amare, E. Enhancing the Photocatalytic Efficiency of ZnO: Defects, Heterojunction, and Optimization. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100336. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the Criteria anticipated for the Fabrication of Highly Efficient ZnO-based Visible-Light-driven Photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Cerrato, E.; Paganini, M.C. Mechanism of Visible Photon Absorption: Unveiling of the C3N4–ZnO Photoactive Interface by means of EPR Spectroscopy. Mater. Adv. 2020, in press. [Google Scholar] [CrossRef]
- Subha, N.; Mahalakshmi, M.; Myilsamy, M.; Neppolian, B.; Murugesan, V. Direct Z-scheme heterojunction nanocomposite for the enhanced solar H 2 production. Appl. Catal. A 2018, 553, 43–51. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-Scheme Photocatalysts: Principles, Synthesis, and Applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 16011694. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Sacco, O.; Sannino, D. Photocatalytic treatment of aqueous solutions at high dye concentration using praseodymium-doped ZnO catalysts. Appl. Catal. B 2017, 209, 621–630. [Google Scholar] [CrossRef]
- Cerrato, E.; Gionco, C.; Berruti, I.; Sordello, F.; Calza, P.; Paganini, M.C. Rare earth ions doped ZnO: Synthesis, characterization and preliminary photoactivity assessment. J. Solid State Chem. 2018, 264, 42–47. [Google Scholar] [CrossRef]
- Sordello, F.; Berruti, I.; Gionco, C.; Paganini, M.C.; Calza, P.; Minero, C. Photocatalytic Performances of Rare Earth Element-Doped Zinc Oxide toward Pollutant Abatement in Water and Wastewater. Appl. Catal. B 2019, 245, 159–166. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Gohain, M.; Kumar, V.; Som, S.; Bezuindenhoudt, B.C.B.; Ntwaeaborwa, O.M. Influence of Ultrasonication Times on the Tunable Colour Emission of ZnO Nanophosphors for Lighting Applications. Ultrason. Sonochem. 2014, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, R.; Lemos, A.F.; Reblo, A.; Ahangar, H.A.; Ferreira, J.M.F. Effects of Rare-Earth (Er, La and Yb) Doping on Morphology and Structure Properties of ZnO Nanostructures Prepared by Wet Chemical Method. Ceram. Int. 2014, 40, 523–529. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Olaru, N.; Samoila, P.; Airinei, A.; Ignat, M.; Sacarescu, L.; Timpu, D. Novel Rare Earth (RE-La, Er, Sm) Metal doped ZnO Photocatalysts for Degradation of Congo-Red dye: Synthesis, Characterization and Kinetic Studies. J. Environ. Manag. 2019, 239, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, E.; Zickler, G.A.; Paganini, M.C. The Role of Yb Doped ZnO in the Charge Transfer Process and Stabilization. J. Alloys Compd. 2019, 816, 152555. [Google Scholar] [CrossRef]
- Chouchene, B.; Ben Chaabane, T.; Balan, L.; Girot, E.; Mozet, K.; Medjahdi, G.; Schneider, R. High Performance Ce-doped ZnO Nanorods for Sunlight-Driven Photocatalysis. Beilstein J. Nanotechnol. 2016, 7, 1338–1349. [Google Scholar] [CrossRef]
- Munawar, T.; Yasmeen, S.; Hasan, M.; Mahmood, K.; Hussain, A.; Ali, A.; Arshad, M.I.; Iqbal, F. Novel Tri-Phase Heterostructured ZnO–Yb2O3–Pr2O3 Nanocomposite; Structural, optical, Photocatalytic and Antibacterial Studies. Ceram. Int. 2020, 46, 11101–11114. [Google Scholar] [CrossRef]
- Calza, P.; Gionco, C.; Giletta, M.; Kalaboka, M.; Sakkas, V.A.; Albanis, T.; Paganini, M.C. Assessment of the Abatement of Acelsulfame K Using Cerium Doped ZnO as Photocatalyst. J. Hazard. Mater. 2016, 323, 471–477. [Google Scholar] [CrossRef]
- Paganini, M.C.; Dalmasso, D.; Gionco, C.; Polliotto, V.; Mantilleri, L.; Calza, P. Beyond TiO2: Cerium-Doped Zinc Oxide as a New Photocatalyst for the Photodegradation of Persistent Pollutants. ChemistrySelect 2016, 1, 3377–3383. [Google Scholar] [CrossRef]
- Bechambi, O.; Jlaiel, L.; Najjar, W.; Sayadi, S. Photocatalytic Degradation of Bisphenol A in the Presence of Ce-ZnO: Evolution of Kinetics, Toxicity and Photodegradation Mechanism. Mater. Chem. Phys. 2016, 173, 95–105. [Google Scholar] [CrossRef]
- Coleman, V.A.; Jagadish, C. Zinc Oxide Bulk, Thin Films and Nanostructures; Pearton, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–20. [Google Scholar]
- Gerward, L.; Olsen, J.S. The High-Pressure Phase of Zincite. J. Synchrotron Radiat. 1995, 2, 233–235. [Google Scholar] [CrossRef]
- Shestakov, M.V.; Baranov, A.N.; Tikhomirov, V.K.; Zubavichus, Y.V.; Kuznetsov, A.S.; Veligzhanin, A.A.; Kharin, A.Y.; Rösslhuber, R.; Timoshenko, V.Y.; Moshchalkov, V.V. Energy-Transfer Luminescence of a Zinc Oxide/Ytterbium Oxide Nanocomposite. RSC Adv. 2012, 2, 8783. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the Optical Band Gap of Zinc Oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Reszczyńska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible Light Activity of Rare Earth Metal Doped (Er3+, Yb3+ or Er3+/Yb3+) Titania Photocatalysts. Appl. Catal. B 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Carnall, W.T. Handbook on the Physics and Chemistry of Rare Earths; Gschneidner, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 1979; Volume 3, pp. 171–208. [Google Scholar]
- Carnall, W.T.; Fields, P.R.; Sarup, R. Optical Absorption Spectra of Er3+:LaF3 and ErCl3 · 6H2O. J. Chem. Phys. 1972, 57, 43–51. [Google Scholar] [CrossRef]
- Cerrato, E.; Gionco, C.; Paganini, M.C.; Giamello, E.; Albanese, E.; Pacchioni, G. Origin of Visible Light Photoactivity of the CeO2/ZnO Heterojunction. ACS Appl. Energy Mat. 2018, 1, 4247–4260. [Google Scholar] [CrossRef]
- Barolo, G.; Livraghi, S.; Chiesa, M.; Paganini, M.C.; Giamello, E. Mechanism of the Photoactivity under Visible Light of N-Doped Titanium Dioxide. Charge Carriers Migration in Irradiated N-TiO2 Investigated by Electron Paramagnetic Resonance. J. Phys. Chem. C 2012, 116, 20887–20894. [Google Scholar] [CrossRef]
- Look, D.C.; Hemsky, J.W. Residual Native Shallow Donor in ZnO. Phys. Rev. Lett. 1999, 82, 2552–2555. [Google Scholar] [CrossRef]
- Halliburton, L.E.; Giles, N.C.; Garces, N.Y.; Luo, M.; Xu, C.; Boatner, L.B.A. Production of Native Donors in ZnO by Annealing at High Temperature in Zn Vapour. Appl. Phys. Lett. 2005, 87, 1721081–1721083. [Google Scholar] [CrossRef]
- Wong, N.B.; Taarit, Y.B.; Lunsford, J.H. Formation of O− in ZnO from the Dissociation of Adsorbed N2O. J. Chem. Phys. 1974, 60, 2148–2151. [Google Scholar] [CrossRef]
- Codell, M.; Gisser, H.; Weisberg, J.; Iyengar, R.D. Electron Spin Resonance Study of Hydroperoxide on Zinc Oxide. J. Phys. Chem. C 1968, 72, 2460–2464. [Google Scholar] [CrossRef]
- Hofmann, D.M.; Hofstaetter, A.; Leiter, F.; Zhou, H.; Henecker, F.; Meyer, B.K.; Orlinskii, S.B.; Schmidt, J.; Baranov, P.G. Hydrogen: A Relevant Shallow Donor in Zinc Oxide. Phys. Rev. Lett. 2002, 88, 0455041–0455044. [Google Scholar] [CrossRef] [PubMed]
- Kohan, A.F.; Ceder, G.; Morgan, D.; Van de Walle, C.G. First-Principles Study of Native Point Defects in ZnO. Phys. Rew. B 2000, 61. [Google Scholar] [CrossRef]
- Walle, C.G.V.d. Hydrogen as a Cause of Doping in Zinc Oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Vehse, W.E. ESR of electron irradiated ZnO confirmation of the F+center. Phys. Lett. 1970, 31A, 147–148. [Google Scholar] [CrossRef]
- Setaka, M.; Fujieda, S.; Kwan, T. Electron Spin Resonance of Oxidized ZnO at -195 °C. Bull. Chem. Soc. Jpn. 1970, 43, 2377–2380. [Google Scholar] [CrossRef]
- Kokes, R.J. The influence of Chemisorption of Oxygen on the Electron Spin Resonance of zinc Oxide. J. Phys. Chem. C 1962, 66, 99–103. [Google Scholar] [CrossRef]
- Schulz, M. ESR Experiments on Ga Donors in ZnO Crystals Phys. Stat. Sol. A 1975, 27, 5–8. [Google Scholar] [CrossRef]
- Gonzalez, C.; Block, D.; Cox, R.T.; Hervè, A. Magnetic Resonance Studies of Shallow Donors in Zinc Oxide. J. Cryst. Growth 1982, 59, 357–362. [Google Scholar] [CrossRef]
- Block, D.; Hervé, A.; Cox, R.T. Optically Detected Magnetic Resonance and Optically Detected ENDOR of Shallow Indium Donors in ZnO. Phys. Rev. B 1982, 25, 6049–6052. [Google Scholar] [CrossRef]
- Meyer, B.K.; Alves, H.; Hofmann, D.M.; Kriegseis, W.; Forster, D.; Bertram, F.; Christen, J.; Hoffmann, A.; Straßburg, M.; Dworzak, M.; et al. Bound Exciton and Donor–Acceptor Pair Recombinations in ZnO. Phys. Stat. Sol. B 2004, 241, 231–260. [Google Scholar] [CrossRef]
- Leutwein, K.; Schneider, J. Defects in Neutron-irradiated ZnO. Z. Naturforsch. 1971, 26a, 1236–1237. [Google Scholar] [CrossRef]
- Gallad, D.; Herve, A. ESR Spectra of the Zinc Vacancy in ZnO. Phys. Lett. 1970, 33A, 1–2. [Google Scholar] [CrossRef]
- Galland, D.; Herve, A. Temperature Ddependance of the ESR Spectrum of The Zinc Vacancy in ZnO. Solid State Commun. 1974, 14, 953–956. [Google Scholar] [CrossRef]
- Che, M.; Tench, A.J. Characterization and Reactivity of Mononuclear Oxygen Species on Oxide Surfaces. Adv. Catal. 1982, 31. [Google Scholar]
- Iyengar, R.D. TiO2 and ZnO Surface Studies by Electron Spin Resonance Spectroscopy. Adv. Colloid Interface Sci. 1972, 3, 365–388. [Google Scholar] [CrossRef]
- Volodin, A.M.; Cherkashin, A.E. ESR Studies of N2O Interaction with Photoinduced Centers on ZnO and MgO React. Kinet. Catal. Lett. 1982, 20, 335–338. [Google Scholar] [CrossRef]
- Volodin, A.M.; Cherkaskin, A.E. ERR Spectrum of Methyl Radicals on ZnO Surface. React. Kinet. Catal. Lett. 1981, 18, 243–246. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constant for reactions of hydrated electrons, hydrogen-atoms and hydroxil radicals (.OH/.O-) in aqueous-solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. Enhanced photocatalytic activity of sulfur doped TiO2 for the decomposition of phenol: A new insight into the bulk and surface modification. Mater. Chem. Phys. 2014, 143, 1300–1308. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.I. Effect of surface hydroxyl groups of pure TiO2 and modified TiO2 on the photocatalytic oxidation of aqueous cyanide. Korean J. Chem. Eng. 2004, 21, 116–122. [Google Scholar] [CrossRef]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A Chem. 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Palominos, R.; Freer, J.; Mondaca, M.A.; Mansilla, H.D. Evidence for hole participation during the photocatalytic oxidation of the antibiotic flumequine. J. Photochem. Photobiol. A Chem. 2008, 193, 139–145. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rao, K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Recio, J.M.; Blanco, M.A.; Luan, V.; Pandey, R.; Gerward, L.; Olsen, J.S. Compressibility of the high-pressure rocksalt phase of ZnO. Phys. Rev. B 1998, 58, 8949–8954. [Google Scholar] [CrossRef]
- Cerrato, E.; Gionco, C.; Paganini, M.C.; Giamello, E. Photoactivity Properties of ZnO Doped with Cerium Ions: An EPR Study. J. Phys. Condens. Matter. 2017, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.W.; Hill, N.J. Electron Paramagnetic Resonance Study of Ce3+, Dy3+ and Yb3+ in Cs2NaYCl6. J. Chem. Soc. Faraday Trans. 2 1974, 70, 124–131. [Google Scholar] [CrossRef]
- Pidol, L.; Guillot-Noël, O.; Kahn-Harari, A.; Viana, B.; Pelenc, D.; Gourier, D. EPR Study of Ce3+ Ions in Lutetium Silicate Scintillators Lu2Si2O7 and Lu2SiO5. J. Phys. Chem. Solids 2006, 67, 643–650. [Google Scholar] [CrossRef]
- Chen, S.F.; Liu, Y.Z. Study on the photocatalytic degradation of glyphosate by TiO2 photocatalyst. Chemosphere 2007, 67, 1010–1017. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, S.; Gui, W.; Zeng, Q. First-Principles Study of Structure, Mechanical and Optical Properties of La- and Sc-doped Y2O3. J. Rare Earths 2019, 37, 879–885. [Google Scholar] [CrossRef]
- Lòpez-Anguillar, F.; Costa-Quintana, J. A First-Principles Pseudopotential Model for the Strong Intrasite Interaction Applied to the 4f13 Configuration (Yb2O3). Phys. Stat. Sol. B 1984, 123, 219. [Google Scholar] [CrossRef]
- Prokofiev, A.V.; Shelykh, A.I.; Melekh, B.T. Periodicity in the band gap variation of Ln2X3 (X = O, S, Se) in the lanthanide series. J. Alloys Compd. 1996, 242, 41–44. [Google Scholar] [CrossRef]




| Sample | Phase | a(Å) | Δa | c(Å) | Δc | d(nm) | Δd |
|---|---|---|---|---|---|---|---|
| ZnO | ZnO | 3.2554 | 0.0000 | 5.2143 | 0.0000 | 256 | 0 |
| CZ1 | ZnO | 3.2528 | −0.0026 | 5.2111 | −0.0032 | 163 | −93 |
| CeO2 | 5.4111 | 10 | |||||
| YZ1 | ZnO | 3.2536 | −0.0019 | 5.2122 | −0.0021 | 93 | −163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 European Union; Licensee MDPI, Basel, Switzerland. This is an open access article distributed under the terms of the European Union License (CC BY) license (http://creativecommons.org/licenses/by/3.0/igo), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any reproduction of this article there should not be any suggestion that European Union or this article endorse any specific organization or products. The use of the European Union logo is not permitted.
Share and Cite
Cerrato, E.; Gonçalves, N.P.F.; Calza, P.; Paganini, M.C. Comparison of the Photocatalytic Activity of ZnO/CeO2 and ZnO/Yb2O3 Mixed Systems in the Phenol Removal from Water: A Mechanicistic Approach. Catalysts 2020, 10, 1222. https://doi.org/10.3390/catal10101222
Cerrato E, Gonçalves NPF, Calza P, Paganini MC. Comparison of the Photocatalytic Activity of ZnO/CeO2 and ZnO/Yb2O3 Mixed Systems in the Phenol Removal from Water: A Mechanicistic Approach. Catalysts. 2020; 10(10):1222. https://doi.org/10.3390/catal10101222
Chicago/Turabian StyleCerrato, Erik, Nuno Paulo Ferreira Gonçalves, Paola Calza, and Maria Cristina Paganini. 2020. "Comparison of the Photocatalytic Activity of ZnO/CeO2 and ZnO/Yb2O3 Mixed Systems in the Phenol Removal from Water: A Mechanicistic Approach" Catalysts 10, no. 10: 1222. https://doi.org/10.3390/catal10101222