Pathologies of Precursor Lesions of Biliary Tract Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Biliary Tract Carcinomas and Precursors as Pancreatobiliary Neoplasms
2.1. Backgrounds
2.1.1. Embryology and Anatomy
2.1.2. Multiple Cell Types Composing the Individual Regions of the Pancreatobiliary System
2.1.3. Four Cell Lineages
2.2. Biliary Tract Carcinomas (BTCs)
2.2.1. Epidemiology
2.2.2. Gross and Microscopic Features of BTCs
2.2.3. Four Subtypes of BTCs as a Spectrum of Pancreatobiliary Carcinoma
2.2.4. LD-iCCA, pCCA, dCCA, and GBC Share Variable Pathologic and Immunohistochemical Features
2.2.5. Genetic Alterations in LD-iCCA, pCCA, dCCA, and GBC
2.3. Precursors of the Pancreatobiliary System
2.3.1. Precursors of the Biliary Tract
2.3.2. Precursors of the Pancreas and Ampulla
2.4. Treatments and Diagnostic Approaches of BTCs and Precursors of the Biliary Tract
3. Common Precursors of the Biliary Tract
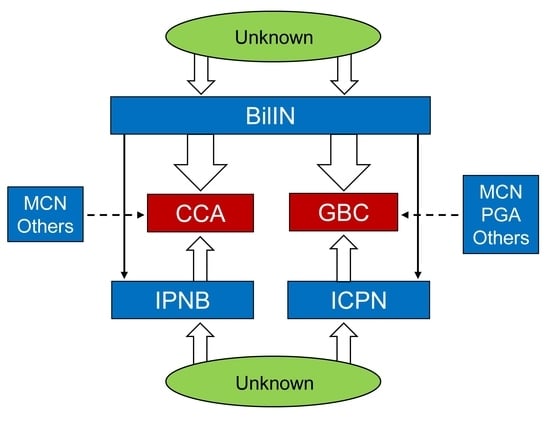
3.1. BilIN
3.1.1. Clinical Features and Background
Clinical Features
Backgrounds
Epidemiology
3.1.2. Gross and Microscopic Features
3.1.3. Grading
3.1.4. Four Subtypes
3.1.5. Invasion
- (i)
- High-BilIN associated with focal stromal invasion
- (ii)
- High-grade BilIN in the surrounding mucosa around invasive CCAs and GBC.
3.1.6. Origin in the Biliary Tract, Pathogenesis, and Molecular and Genetic Changes
- (a)
- Origins in the biliary tract
- (b)
- Pathogenesis
- Background lesions
- 2.
- Molecular and genetic changes
3.1.7. Staging and Prognosis
- (a)
- Staging
- (b)
- Prognosis
3.1.8. Related Diseases and Differential Diagnosis
- (1)
- IPNB and ICPN
- (2)
- “Intraductal spread of invasive carcinoma (cancerization)”
- (3)
- Reactive atypia
3.2. IPNB
3.2.1. Clinical Features and Background and Epidemiology
3.2.2. Gross and Microscopic Features and Intraepithelial Spread and Glandular Involvement
3.2.3. Pathological Grading: Modified Two-Tiered Grading (Type 1 and Type 2 Subclassification)
3.2.4. Subtypes Based on Cell Lineage
- (i)
- Intestinal subtype
- (ii)
- Gastric subtype
- (iii)
- Pancreatobiliary subtype
- (iv)
- Oncocytic subtype
3.2.5. Invasion and Multiple Occurrence
3.2.6. Pathogenesis and Molecular Genetic Alterations
3.2.7. Staging and Prognosis
3.2.8. Related Diseases and Differential Diagnosis
3.3. ICPN
3.3.1. Clinicopathological Features and Background and Epidemiology
3.3.2. Gross and Microscopic Features and Intraepithelial Spreading
Gross and Microscopic Findings
3.3.3. Pathological Grading: Two-Tiered Grading System
3.3.4. Subtypes Based on Cell Lineage
3.3.5. Invasion
3.3.6. Pathogenesis and Molecular and Genetic Changes
3.3.7. Staging (TNM) and Prognosis
3.3.8. Related Diseases and Differential Diagnosis
Related Disease
Differential Diagnosis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Digestive System Tumours. In WHO Classification of Tumours, 5th ed.; International Agency for Reearch on Cancer: Lyon, France, 2019. [Google Scholar]
- Moeini, A.; Haber, P.K.; Sia, D. Cell of origin in biliary tract cancers and clinical implications. JHEP Rep. 2021, 3, 100226. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yeh, M.M. Bile duct dysplasia and associated invasive carcinoma: Clinicopathological features, diagnosis, and practical challenges. Hum. Pathol. 2022; online ahead of print. [Google Scholar]
- Albores-Saavedra, J.; Murakata, L.; Krueger, J.E.; Henson, D.E. Noninvasive and minimally invasive papillary carcinomas of the extrahepatic bile ducts. Cancer 2000, 89, 508–515. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Terada, T.; Tanaka, Y.; Ohta, G. Are hepatolithiasis and cholangiocarcinoma aetiologically related? A morphological study of 12 cases of hepatolithiasis associated with cholangiocarcinoma. Virchows Arch. A Pathol. Anat. Histopathol. 1985, 406, 45–58. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Uesaka, K.; Kakuda, Y.; Sugino, T.; Kubota, K.; Furukawa, T.; Fukumura, Y.; Isayama, H.; Terada, T. Intraductal papillary neoplasm of bile duct: Updated clinicopathological characteristics and molecular and genetic alterations. J. Clin. Med. 2020, 9, 3991. [Google Scholar] [CrossRef]
- Gordon-Weeks, A.N.; Jones, K.; Harriss, E.; Smith, A.; Silva, M. Systematic review and meta-analysis of current experience in treating IPNB: Clinical and pathological correlates. Ann. Surg. 2016, 263, 656–663. [Google Scholar] [CrossRef]
- Saei Hamedani, F.; Garcia-Buitrago, M. Intracholecystic Papillary-Tubular Neoplasms (ICPN) of the Gallbladder: A Short Review of Literature. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 57–61. [Google Scholar] [CrossRef]
- Zen, Y. Intrahepatic cholangiocarcinoma: Typical features, uncommon variants, and controversial related entities. Hum. Pathol. 2022; online ahead of print. [Google Scholar]
- Singh, N.; Zaidi, A.; Kaur, R.; Kaur, J.; Nijhawan, V.S. Incidental gallbladder neoplasms: A growing global burden. Cureus 2022, 14, e25805. [Google Scholar] [CrossRef]
- Sato, Y.; Harada, K.; Sasaki, M.; Nakanuma, Y. Histological characterization of biliary intraepithelial neoplasia with respect to pancreatic intraepithelial neoplasia. Int. J. Hepatol. 2014, 2014, 678260. [Google Scholar] [CrossRef] [Green Version]
- Nakanuma, Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: Is the biliary tract an incomplete pancreas? Pathol. Int. 2010, 60, 419–429. [Google Scholar] [CrossRef]
- Schlitter, A.M.; Born, D.; Bettstetter, M.; Specht, K.; Kim-Fuchs, C.; Riener, M.O.; Jeliazkova, P.; Sipos, B.; Siveke, J.T.; Terris, B.; et al. Intraductal papillary neoplasms of the bile duct: Stepwise progression to carcinoma involves common molecular pathways. Mod. Pathol. 2014, 27, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Sasaki, M.; Harada, K.; Aishima, S.; Fukusato, T.; Ojima, H.; Kanai, Y.; Kage, M.; Nakanuma, Y.; Tsubouchi, H. Pathological diagnosis of flat epithelial lesions of the biliary tract with emphasis on biliary intraepithelial neoplasia. J. Gastroenterol. 2014, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Fernández Moro, C.; Fernandez-Woodbridge, A.; Alistair D’souza, M.; Zhang, Q.; Bozoky, B.; Kandaswamy, S.V.; Catalano, P.; Heuchel, R.; Shtembari, S.; Del Chiaro, M.; et al. Immunohistochemical typing of adenocarcinomas of the pancreatobiliary system improves diagnosis and prognostic stratification. PLoS ONE 2016, 11, e0166067. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.D.; Balci, S.; Ohike, N.; Xue, Y.; Kim, G.E.; Tajiri, T.; Memis, B.; Coban, I.; Dolgun, A.; Krasinskas, A.M.; et al. Ampullary carcinoma is often of mixed or hybrid histologic type: An analysis of reproducibility and clinical relevance of classification as pancreatobiliary versus intestinal in 232 cases. Mod. Pathol. 2016, 29, 1575–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adsay, V.; Jang, K.T.; Roa, J.C.; Dursun, N.; Ohike, N.; Bagci, P.; Basturk, O.; Bandyopadhyay, S.; Cheng, J.D.; Sarmiento, J.M.; et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are ≥1.0 cm): Clinicopathologic and immunohistochemical analysis of 123 cases. Am. J. Surg. Pathol. 2012, 36, 1279–1301. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, G.; Szekerczés, T.; Illyés, I.; Dóra, R.; Kontsek, E.; Gógl, A.; Kiss, A.; Werling, K.; Kovalszky, I.; Schaff, Z.; et al. Cholangiocarcinoma: Classification, histopathology and molecular carcinogenesis. Pathol. Oncol. Res. 2020, 26, 3–15. [Google Scholar] [CrossRef]
- Kendall, T.; Verheij, J.; Gaudio, E.; Evert, M.; Guido, M.; Goeppert, B.; Carpino, G. Anatomical, histomorphologicaland molecular classification of cholangiocarcinoma. Liver Int. 2019, 39, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Fukumura, Y.; Rong, L.; Maimaitiaili, Y.; Fujisawa, T.; Isayama, H.; Nakahodo, J.; Kikuyama, M.; Yao, T. Precursor lesions of gallbladder carcinoma: Disease concept, pathology, and genetics. Diagnostics 2022, 12, 341. [Google Scholar] [CrossRef]
- Roa, J.C.; Basturk, O.; Adsay, V. Dysplasia and carcinoma of the gallbladder: Pathological evaluation, sampling, differential diagnosis and clinical implications. Histopathology 2021, 79, 2–19. [Google Scholar] [CrossRef]
- Carpino, G.; Cardinale, V.; Onori, P.; Franchitto, A.; Berloco, P.B.; Rossi, M.; Wang, Y.; Semeraro, R.; Anceschi, M.; Brunelli, R.; et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: An anatomical in situ study yielding evidence of maturational lineages. J. Anat. 2012, 220, 186–199. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Sudo, Y. Biliary tumors with pancreatic counterparts. Semin. Diagn. Pathol. 2017, 34, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Cardinale, V.; Carpino, G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatology 2016, 64, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.T.; Ahn, S. Tumoral versus flat intraepithelial neoplasia of pancreatobiliary tract, gallbladder, and ampulla of Vater. Arch. Pathol. Lab. Med. 2016, 140, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Gandou, C.; Harada, K.; Sato, Y.; Igarashi, S.; Sasaki, M.; Ikeda, H.; Nakanuma, Y. Hilar cholangiocarcinoma and pancreatic ductal adenocarcinoma share similar histopathologies, immunophenotypes, and development-related molecules. Hum. Pathol. 2013, 44, 811–821. [Google Scholar] [CrossRef]
- Zen, Y.; Hubscher, S.G.; Nakanuma, Y. Bile duct diseases. In MacSween’s Pathology of the Liver; Elsevier: Amsterdam, The Netherlands, 2018; pp. 515–593. [Google Scholar]
- Dong, P.D.; Munson, C.A.; Norton, W.; Crosnier, C.; Pan, X.; Gong, Z.; Neumann, C.J.; Stainier, D.Y. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat. Genet. 2007, 39, 397–402. [Google Scholar] [CrossRef]
- Delous, M.; Yin, C.; Shin, D.; Ninov, N.; Debrito Carten, J.; Pan, L.; Ma, T.P.; Farber, S.A.; Moens, C.B.; Stainier, D.Y.; et al. Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS Genet. 2012, 8, e1002754. [Google Scholar] [CrossRef] [Green Version]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 2018, 173, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, J.; Mino-Kenudson, M.; Liss, A.S.; Chowdhury, S.; Wang, T.C.; Fernández-Del Castillo, C.; Lillemoe, K.D.; Warshaw, A.L.; Thayer, S.P. Loss of Trefoil Factor 2 from pancreatic duct glands promotes formation of intraductal papillary mucinous neoplasms in mice. Gastroenterology 2016, 151, 1232–1244. [Google Scholar] [CrossRef] [Green Version]
- Terada, T.; Nakanuma, Y.; Kakita, A. Pathologic observations of intrahepatic peribiliary glands in 1000 consecutive autopsy livers. Heterotopic pancreas in the liver. Gastroenterology 1990, 98, 1333–1337. [Google Scholar] [CrossRef]
- Lack, E.E. Normal anatomy of the gallbladder and cystic duct along with developmental and other abnormalities. In Pathology of the Pancreas, Gallbladder, Extrahepatic Biliary Tract, and Ampullary Region; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Hayata, Y.; Nakagawa, H.; Kurosaki, S.; Kawamura, S.; Matsushita, Y.; Hayakawa, Y.; Suzuki, N.; Hata, M.; Tsuboi, M.; Kinoshita, H.; et al. Axin2+ peribiliary glands in the periampullary region generate biliary epithelial stem cells that give rise to ampullary carcinoma. Gastroenterology 2021, 160, 2133–2148. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hayata, Y.; Yamada, T.; Kawamura, S.; Suzuki, N.; Koike, K. Peribiliary glands as the cellular origin of biliary tract cancer. Int. J. Mol. Sci. 2018, 19, 1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terada, T.; Nakanuma, Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers. II. A possible source of cholangiocarcinoma. Hepatology 1990, 12, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Uesaka, K.; Okamura, Y.; Terada, T.; Fukumura, Y.; Kakuda, Y.; Sugino, T.; Sato, Y.; Take, J.K.; Park, Y.N. Reappraisal of pathological features of intraductal papillary neoplasm of bile duct with respect to the type 1 and 2 subclassifications. Hum. Pathol. 2021, 111, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Rouzbahman, M.; Serra, S.; Adsay, N.V.; Bejarano, P.A.; Nakanuma, Y.; Chetty, R. Oncocytic papillary neoplasms of the biliary tract: A clinicopathological, mucin core and Wnt pathway protein analysis of four cases. Pathology 2007, 39, 413–418. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Uesaka, K.; Terada, T.; Fukumura, Y.; Sugino, T.; Kakuda, Y.; Ikeda, H.; Harada, K.; Sato, Y.; Shimizu, S.; et al. Gastric subtype of intraductal papillary neoplasm of the bile duct: The pathologic spectrum. J. Hepatobiliary Pancreat. Sci. 2020, 7, 402–413. [Google Scholar] [CrossRef]
- Bronsert, P.; Kohler, I.; Werner, M.; Makowiec, F.; Kuesters, S.; Hoeppner, J.; Hopt, U.T.; Keck, T.; Bausch, D.; Wellner, U.F. Intestinal-type of differentiation predicts favourable overall survival: Confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin. BMC Cancer 2013, 13, 428. [Google Scholar] [CrossRef] [Green Version]
- Brierley, J.D.; Gospodarowicz, M.; Witterkind, C. TNM Classification of Malignant Tumours, 8th ed.; UICC International Union Against Cancer: Geneva, Switzerland, 2017. [Google Scholar]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Bertran, E.; Heise, K.; Andia, M.E.; Ferreccio, C. Gallbladder cancer: Incidence and survival in a high-risk area of Chile. Int. J. Cancer 2010, 127, 2446–2454. [Google Scholar] [CrossRef]
- Hundal, R.; Shaffer, E.A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014, 6, 99–109. [Google Scholar]
- Bridgewater, J.A.; Goodman, K.A.; Kalyan, A.; Mulcahy, M.F. Biliary tract cancer: Epidemiology, gadiotherapy, and molecular profiling. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e194–e203. [Google Scholar] [CrossRef]
- Japanese Society of Hepato-Biliary-Pancreatic Surgery. General Rules for Clinical and Pathological Studies on Cancer of the Biliary Tree, 7th ed.; Kanehara Pub: Tokyo, Japan, 2021. (In Japanese) [Google Scholar]
- Nguyen Canh, H.; Takahashi, K.; Yamamura, M.; Li, Z.; Sato, Y.; Yoshimura, K.; Kozaka, K.; Tanaka, M.; Nakanuma, Y.; Harada, K. Diversity in cell differentiation, histology, phenotype and vasculature of mass-forming intrahepatic cholangiocarcinomas. Histopathology 2021, 79, 731–750. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kubota, K.; Hachiya, H.; Sakuraoka, Y.; Shiraki, T.; Shimizu, T.; Mori, S.; Iso, Y.; Kato, M.; Yamagishi, H.; et al. Impact of tumor location on postoperative outcome of intraductal papillary neoplasm of the bile duct. World J. Surg. 2019, 43, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Westgaard, A.; Tafjord, S.; Farstad, I.N.; Cvancarova, M.; Eide, T.J.; Mathisen, O.; Clausen, O.P.F.; Gladhaug, I.P. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer 2008, 8, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, W.; Futakawa, N.; Yamagata, S.; Wada, Y.; Kuroda, A.; Muto, T.; Esaki, Y. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J. Cancer Res. 1994, 85, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Westgaard, A.; Pomianowska, E.; Clausen, O.P.; Gladhaug, I.P. Intestinal-type and pancreatobiliary-type adenocarcinomas: How does ampullary carcinoma differ from other periampullary malignancies? Ann. Surg. Oncol. 2013, 20, 430–439. [Google Scholar] [CrossRef]
- Akita, M.; Sofue, K.; Fujikura, K.; Otani, K.; Itoh, T.; Ajiki, T.; Fukumoto, T.; Zen, Y. Histological and molecular characterization of intrahepatic bile duct cancers suggests an expanded definition of perihilar cholangiocarcinoma. HPB 2019, 21, 226–234. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Bizama, C.; García, P.; Ferreccio, C.; Javle, M.; Miquel, J.F.; Koshiol, J.; Roa, J.C. The inflammatory inception of gallbladder cancer. Biochim. Biophys. Acta 2016, 1865, 245–254. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; Elzawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef]
- Jiao, Y.; Pawlik, T.M.; Anders, R.A.; Selaru, F.M.; Streppel, M.M.; Lucas, D.J.; Niknafs, N.; Guthrie, V.B.; Maitra, A.; Argani, P.; et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat. Genet. 2013, 45, 1470–1473. [Google Scholar] [CrossRef] [Green Version]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Shibata, T.; Arai, Y.; Totoki, Y. Molecular genomic landscapes of hepatobiliary cancer. Cancer Sci. 2018, 109, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Wardell, C.P.; Fujita, M.; Yamada, T.; Simbolo, M.; Fassan, M.; Karlic, R.; Polak, P.; Kim, J.; Hatanaka, Y.; Maejima, K.; et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J. Hepatol. 2018, 68, 959–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazcano-Ponce, E.C.; Miquel, J.F.; Muñoz, N.; Herrero, R.; Ferrecio, C.; Wistuba, I.I.; Alonso de Ruiz, P.; Aristi Urista, G.; Nervi, F.C.A. Epidemiology and molecular pathology of gallbladder cancer. Cancer J. Clin. 2001, 51, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Adsay, N.V.; Albores-Saavedra, J.; Compton, C.; Garrett, E.S.; Goodman, S.N.; Kern, S.E.; Klimstra, D.S.; Klöppel, G.; Longnecker, D.S.; et al. Pancreatic intrae-pithelial neoplasia: A new nomenclature and classification system for pancreatic duct lesions. Am. J. Surg. Pathol. 2001, 25, 579–586. [Google Scholar] [CrossRef]
- Bickenbach, K.; Galka, E.; Roggin, K.K. Molecular mechanisms of cho-langiocarcinogenesis: Are biliary intraepithelial neoplasia and intraductal papillary neoplasms of the bile duct precursors to cholan-giocarcinoma? Surg. Oncol. Clin. N. Am. 2009, 18, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Kakuda, Y. Pathologic classification of cholangiocarcinoma: New concepts. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 277–293. [Google Scholar] [CrossRef]
- Makuchi, M.; Kokudo, N.; Popescu, I.; Belghiti, J.; Han, H.S.; Kakaori, K.; Duda, D.G. The IASGO Textbook of Multi-Disciplinary Management of Hepato-Pancreato-Biliary Diseases; International Association of Surgeons, Gastroenterologists and Oncologists, Ed.; Springer: Singapore, 2022; p. 548. [Google Scholar]
- Aishima, S.; Iguchi, T.; Fujita, N.; Taketomi, A.; Maehara, Y.; Tsuneyoshi, M.; Oda, Y. Histological and immunohistological findings in biliary intraepithelial neoplasia arising from a background of chronic biliary disease compared with liver cirrhosis of non-biliary aetiology. Histopathology 2011, 59, 867–875. [Google Scholar] [CrossRef]
- Wu, T.T.; Levy, M.; Correa, A.M.; Rosen, C.B.; Abraham, S.C. Biliary intraepithelial neoplasia in patients without chronic biliary disease: Analysis of liver explants with alcoholic cirrhosis, hepatitis C infection, and noncirrhotic liver diseases. Cancer 2009, 115, 4564–4575. [Google Scholar] [CrossRef]
- Zen, Y.; Adsay, N.V.; Bardadin, K.; Colombari, R.; Ferrell, L.; Haga, H.; Hong, S.M.; Hytiroglou, P.; Klöppel, G.; Lauwers, G.Y.; et al. Biliary intraepithelial neoplasia: An international interobserver agreement study and proposal for diagnostic criteria. Mod. Pathol. 2007, 20, 701–709. [Google Scholar] [CrossRef] [Green Version]
- Katabi, N.; Albores-Saavedra, J. The extrahepatic bile duct lesions in end-stage primary sclerosing cholangitis. Am. J. Surg. Pathol. 2003, 27, 349–355. [Google Scholar] [CrossRef]
- Lewis, J.T.; Talwalkar, J.A.; Rosen, C.B.; Smyrk, T.C.; Abraham, S.C. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: Evidence for a metaplasia-dysplasia-carcinoma sequence. Am. J. Surg. Pathol. 2007, 31, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, K.; Kawaguchi, S.; Doi, M.; Mizuno, W.; Uragami, K.; Oka, T.; Miyamoto, H.; Itoh, H.; Nishioka, S. A case of primary intrahepatic sclerosing cholangitis (PISC) complicated with atypical biliary epithelial proliferation. Intern. Med. 1992, 31, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanuma, Y.; Sugino, T.; Okamura, Y.; Nomura, Y.; Watanabe, H.; Terada, T.; Sato, Y. Characterization of high-grade biliary intraepithelial neoplasm of the gallbladder in comparison with intracholecystic papillary neoplasm. Hum. Pathol. 2021, 116, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, H.; Lingohr, P.; Strässer, A.; Dietrich, D.; Rostamzadeh, B.; Glees, S.; Roering, M.; Möhring, P.; Scheerbaum, M.; Stoffels, B.; et al. Biliary intraepithelial neoplasia (BilIN) is frequently found in surgical margins of biliary tract cancer resection specimens but has no clinical implications. Virchows Arch. 2015, 466, 133–141. [Google Scholar] [CrossRef]
- Yoon, K.C.; Yu, Y.D.; Kang, W.H.; Jo, H.S.; Kim, D.S.; Kim, J.Y. Prevalence and clinical significance of biliary intraepithelial neoplasia (BilIN) in cholangiocarcinoma. Am. Surg. 2019, 85, 511–517. [Google Scholar] [CrossRef]
- Shin, D.; Lee, S.; Lee, J.H.; Hong, S.M.; Park, S.Y.; Yoo, C.; Lee, W.; Song, K.B.; Hwang, D.W.; Kim, S.C. Prognostic implication of high grade biliary intraepithelial neoplasia in bile duct resection margins in patients with resected perihilar cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2020, 27, 604–613. [Google Scholar] [CrossRef]
- Basturk, O.; Hong, S.M.; Wood, L.D.; Adsay, N.V.; Albores-Saavedra, J.; Biankin, A.V.; Brosens, L.A.; Fukushima, N.; Goggins, M.; Hruban, R.H.; et al. A Revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am. J. Surg. Pathol. 2015, 39, 1730–1741. [Google Scholar] [CrossRef]
- Yamasaki, S.; Suda, K.; Nobukawa, B.; Sonoue, H. Intraductal spread of pancreatic cancer. Clinicopathologic study of 54 pancreatectomized patients. Pancreatology 2002, 2, 407–412. [Google Scholar] [CrossRef]
- Tanase, H.; Suda, K.; Yamasaki, S.; Nobukawa, B. Intraductal low papillary histological pattern of carcinoma component shows intraductal spread in invasive carcinoma of the pancreas. J. Hepatobiliary Pancreat. Surg. 2006, 13, 235–238. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Kondo, S.; Zen, Y.; Yonemori, A.; Kubota, K.; Kawakami, H.; Tanaka, E.; Hirano, S.; Itoh, T.; Nakanuma, Y. Impact of residual in situ carcinoma on postoperative survival in 125 patients with extrahepatic bile duct carcinoma. J. Hepatobiliary Pancreat. Sci. 2010, 17, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.W. Review of 253 cases of significant pathology in 7,910 cholecystectomies in Hong Kong. Pathology 1988, 20, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Albores-Saavedra, J.; Henson, D.E.; Klimstra, D.S. Tumors of the gallbladder, extrahepatic bile ducts, and Vaterian System. In AFIP Atlas of Tumor Pathology; Series 4; American Registry of Pathology: Silver Spring, MD, USA, 2015; 614p. [Google Scholar]
- Mills, S.E.; Greenson, J.K.; Hornick, J.L.; Longace, J.A.; Reuter, V.E.; Homic, J.L. Sternberg’s Diagnostic Surgical Pathology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015. [Google Scholar]
- Nakanuma, Y.; Sugino, T.; Nomura, Y.; Watanabe, H.; Terada, T.; Sato, Y.; Ohnishi, Y. Association of precursors with invasive adenocarcinoma of the gallbladder: A clinicopathological study. Ann. Diagn. Pathol. 2022, 58, 151911. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Uchida, T.; Sato, Y.; Uesaka, K. An S100P-positive biliary epithelial field is a preinvasive intraepithelial neoplasm in nodular-sclerosing cholangiocarcinoma. Hum. Pathol. 2017, 60, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Albores-Saavedra, J.; Shukla, D.; Carrick, K.; Henson, D.E. In situ and invasive adenocarcinomas of the gallbladder extending into or arising from Rokitansky-Aschoff sinuses: A clinicopathologic study of 49 cases. Am. J. Surg. Pathol. 2004, 28, 621–628. [Google Scholar] [CrossRef]
- Dorantes-Heredia, R.; Chablé-Montero, F.; Henson, D.E.; Albores-Saavedra, J. Rokitansky-Aschoff sinuses mimicking adenocarcinoma of the gallbladder: A study of 8 cases. Am. J. Surg. Pathol. 2013, 37, 1269–1274. [Google Scholar] [CrossRef]
- Aishima, S.; Oda, Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: Different characters of perihilar large duct type versus peripheral small duct type. J. Hepatobiliary Pancreat. Sci. 2015, 22, 94–100. [Google Scholar] [CrossRef]
- Tsuchikawa, T.; Kondo, S.; Hirano, S.; Tanaka, E.; Kato, K.; Matsumoto, J.; Kubota, K.C.; Shichinohe, T. Extensively spreading intraepithelial bile duct carcinoma causing multiple bile duct strictures: Report of three cases. Surg. Today 2011, 41, 1674–1679. [Google Scholar] [CrossRef]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef]
- Bernstein, C.; Bernstein, H.; Payne, C.M.; Dvorak, K.; Garewal, H. Field defects in progression to gastrointestinal tract cancers. Cancer Lett. 2008, 260, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Peng, X.; Dong, K.; Long, J.; Guo, X.; Li, H.; Bai, Y.; Yang, X.; Wang, D.; Lu, X.; et al. Genomic characterization of co-existing neoplasia and carcinoma lesions reveals distinct evolutionary paths of gallbladder cancer. Nat. Commun. 2021, 12, 4753. [Google Scholar] [CrossRef]
- Vij, M.; Puri, Y.; Rammohan, A.; Gowripriya, G.; Rajalingam, R.; Kaliamoorthy, I.; Rela, M. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J. Gastrointest. Oncol. 2022, 14, 607–627. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Gazdar, A.F.; Roa, I.; Albores-Saavedra, J. p53 protein overexpression in gallbladder carcinoma and its precursor lesions: An immunohistochemical study. Hum. Pathol. 1996, 27, 360–365. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Zen, Y.; Kondo, S.; Itoh, T.; Itatsu, K.; Nakanuma, Y. Expression of cell cycle-related molecules in biliary premalignant lesions: Biliary intraepithelial neoplasia and biliary intraductal papillary neoplasm. Hum. Pathol. 2008, 39, 1153–1161. [Google Scholar] [CrossRef]
- Sasaki, M.; Nitta, T.; Sato, Y.; Nakanuma, Y. Autophagy may occur at an early stage of cholangiocarcinogenesis via biliary intraepithelial neoplasia. Hum. Pathol. 2015, 46, 202–209. [Google Scholar] [CrossRef]
- Kubo, Y.; Aishima, S.; Tanaka, Y.; Shindo, K.; Mizuuchi, Y.; Abe, K.; Shirabe, K.; Maehara, Y.; Honda, H.; Oda, Y. Different expression of glucose transporters in the progression of intrahepatic cholangiocarcinoma. Hum. Pathol. 2014, 45, 1610–1617. [Google Scholar]
- Sasaki, M.; Yamaguchi, J.; Itatsu, K.; Ikeda, H.; Nakanuma, Y. Over-expression of polycomb group protein EZH2 relates to decreased expression of p16 INK4a in cholangiocarcinogenesis in hepatolithiasis. J. Pathol. 2008, 215, 175–183. [Google Scholar] [CrossRef]
- Hsu, M.; Sasaki, M.; Igarashi, S.; Sato, Y.; Nakanuma, Y. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer 2013, 119, 1669–1674. [Google Scholar] [CrossRef]
- Walter, D.; Herrmann, E.; Winkelmann, R.; Albert, J.G.; Liese, J.; Schnitzbauer, A.; Zeuzem, S.; Hansmann, M.L.; Peveling-Oberhag, J.; Hartmann, S. Role of CD15 expression in dysplastic and neoplastic tissue of the bile duct—A potential novel tool for differential diagnosis of indeterminate biliary stricture. Histopathology 2016, 69, 962–970. [Google Scholar] [CrossRef]
- Loeffler, M.A.; Hu, J.; Kirchner, M.; Wei, X.; Xiao, Y.; Albrecht, T.; De La Torre, C.; Sticht, C.; Banales, J.M.; Vogel, M.N.; et al. miRNA profiling of biliary intraepithelial neoplasia reveals stepwise tumorigenesis in distal cholangiocarcinoma via the miR-451a/ATF2 axis. J. Pathol. 2020, 252, 239–251. [Google Scholar]
- Kim, Y.T.; Kim, J.; Jang, Y.H.; Lee, W.J.; Ryu, J.K.; Park, Y.K.; Kim, S.W.; Kim, W.H.; Yoon, Y.B.; Kim, C.Y. Genetic alterations in gallbladder adenoma, dysplasia and carcinoma. Cancer Lett. 2001, 169, 59–68. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Maitra, R.; Carraso, R.; Tang, M.; Troncoso, P.; Minna, J.D.; Gazdar, A.F. High resolution chromosome 3p, 8p, 9q and 22q allelotyping analysis in the pathogenesis of gallbladder carcinoma. Br. J. Cancer 2002, 87, 432–440. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.; Mohapatra, T.; Das, P.; Misra, M.C.; Gupta, M.; Ghosh, M.; Kabra, M.; Bansail, V.K.; Kuma, S.; Sreenivias, V.; et al. Sequential occurrence of preneoplastic lesions and accumulation of loss of heterozygosity in patients with gallbladder stones suggest causal association with gallbladder cancer. Ann. Surg. 2016, 260, 1073–1080. [Google Scholar] [CrossRef]
- Koda, M.; Yashima, K.; Kawaguchi, K.; Andachi, H.; Hosoda, A.; Shiota, G.; Ito, H.; Murawaki, Y. Expression of Fhit, Mlh1, and P53 protein in human gallbladder carcinoma. Cancer Lett. 2003, 199, 131–138. [Google Scholar] [CrossRef]
- Wistuba, I.I.; Ashfaq, R.; Maitra, A.; Alvarez, H.; Riquelme, E.; Gazdar, A.F. Fragile histidine triad gene abnormalties in the pathogenesis of gallbladder carcinoma. Am. J. Pathol. 2002, 160, 2073–2079. [Google Scholar] [CrossRef] [Green Version]
- Nagao, M.; Fukuda, A.; Omatsu, M.; Namikawa, M.; Sono, M.; Fukunaga, Y.; Masuda, T.; Araki, O.; Yoshikawa, T.; Ogawa, S.; et al. Concurrent activation of Kras and canonical Wnt signaling induces premalignant lesions that progress to extrahepatic biliary cancer in mice. Cancer Res. 2022, 82, 1803–1817. [Google Scholar] [CrossRef]
- Chang, H.J.; Kim, S.W.; Kim, Y.T.; Kim, W.H. Loss of heterozygosity in dysplasia and carcinoma of the gallbladder. Mod. Pathol. 1999, 12, 763–769. [Google Scholar]
- Roa, J.C.; Tapia, O.; Manterola, C.; Villaseca, M.; Guzman, P.; Araya, J.C.; Bagci, P.; Saka, B.; Adsay, V. Early gallbladder carcinoma has a favorable outcome but Rokitansky-Aschoff sinus involvement is an adverse prognostic factor. Virchows Arch. 2013, 463, 651–661. [Google Scholar] [CrossRef]
- Higuchi, R.; Yazawa, T.; Uemura, S.; Izumo, W.; Furukawa, T.; Yamamoto, M. High-grade dysplasia/carcinoma in situ of the bile duct margin in patients with surgically resected node-negative perihilar cholangiocarcinoma is associated with poor survival: A retrospective study. J. Hepatobiliary Pancreat. Sci. 2017, 24, 456–465. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Jang, K.T.; Fukushima, N.; Furukawa, T.; Hong, S.M.; Kim, H.; Lee, K.B.; Zen, Y.; Jang, J.Y.; Kubota, K. A statement by the Japan-Korea expert pathologists for future clinicopathological and molecular analyses toward consensus building of intraductal papillary neoplasm of the bile duct through several opinions at the present stage. J. Hepatobiliary Pancreat. Sci. 2018, 25, 181–187. [Google Scholar] [CrossRef]
- Kubota, K.; Jang, J.Y.; Nakanuma, Y.; Jang, K.T.; Haruyama, Y.; Fukushima, N.; Furukawa, T.; Hong, S.M.; Sakuraoka, Y.; Kim, H.; et al. Clinicopathological characteristics of intraductal papillary neoplasm of the bile duct: A Japan-Korea collaborative study. J. Hepatobiliary Pancreat. Sci. 2020, 27, 581–597. [Google Scholar]
- Tireli, M.; Uslu, A. Multiple biliary papillomatosis. HPB Surg. 1992, 6, 125–127; discussion 127–128. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuka, M.; Kimura, F.; Shimizu, H.; Yoshidome, H.; Kato, A.; Yoshitomi, H.; Furukawa, K.; Mitsuhashi, N.; Takeuchi, D.; Takayashiki, T.; et al. Surgical strategy for mucin-producing bile duct tumor. J. Hepatobiliary Pancreat. Sci. 2010, 17, 236–240. [Google Scholar] [CrossRef]
- Shibahara, H.; Tamada, S.; Goto, M.; Oda, K.; Nagino, M.; Nagasaka, T.; Batra, S.K.; Hollingsworth, M.A.; Imai, K.; Nimura, Y.; et al. Pathologic features of mucin-producing bile duct tumors: Two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am. J. Surg. Pathol. 2004, 28, 327–338. [Google Scholar] [CrossRef]
- Singhi, A.D.; Wood, L.D.; Parks, E.; Torbenson, M.S.; Felsenstein, M.; Hruban, R.H.; Nikiforova, M.N.; Wald, A.I.; Kaya, C.; Nikiforov, Y.E.; et al. Recurrent rearrangements in PRKACA and PRKACB in intraductal oncocytic papillary neoplasms of the pancreas and bile duct. Gastroenterology 2020, 158, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Nakanuma, Y.; Uesaka, K.; Miyayama, S.; Yamaguchi, H.; Ohtsuka, M. Intraductal neoplasms of the bile duct. A new challenge to biliary tract tumor pathology. Histol. Histopathol. 2017, 32, 1001–1015. [Google Scholar]
- Kubota, K.; Nakanuma, Y.; Kondo, F.; Hachiya, H.; Miyazaki, M.; Nagino, M.; Yamamoto, M.; Isayama, H.; Tabata, M.; Kinoshita, H.; et al. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: A multi-institutional study by the Japan Biliary Association. J. Hepatobiliary Pancreat. Sci. 2014, 21, 176–185. [Google Scholar] [CrossRef]
- Chen, T.C.; Nakanuma, Y.; Zen, Y.; Chen, M.F.; Jan, Y.Y.; Yeh, T.S.; Chiu, C.T.; Kuo, T.T.; Kamiya, J.; Oda, K.; et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology 2001, 34, 651–658. [Google Scholar] [CrossRef]
- Youn, J.M.; Hwang, S.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Hong, S.M. Clinicopathological features and long-term outcomes of intraductal papillary neoplasms of the bile duct of the liver: Single-institution experience with 146 patients. J. Gastrointest. Surg. 2022, 26, 1394–1405. [Google Scholar] [CrossRef]
- Zen, Y.; Jang, K.T.; Ahn, S.; Kim, D.H.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Yeh, M.M. Intraductal papillary neoplasms and mucinous cystic neoplasms of the hepatobiliary system: Demographic differences between Asian and Western populations, and comparison with pancreatic counterparts. Histopathology 2014, 65, 164–173. [Google Scholar] [CrossRef]
- Luvira, V.; Somsap, K.; Pugkhem, A.; Eurboonyanun, C.; Luvira, V.; Bhudhisawasdi, V.; Pairojkul, C.; Ard, S.K. Morphological classification of intraductal papillary neoplasm of the bile duct with survival correlation. Asian Pac. J. Cancer Prev. 2017, 18, 207–213. [Google Scholar]
- Rocha, F.G.; Lee, H.; Katabi, N.; DeMatteo, R.P.; Fong, Y.; D’Angelica, M.I.; Allen, P.J.; Klimstra, D.S.; Jarnagin, W.R. Intraductal papillary neoplasm of the bile duct: A biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 2012, 56, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, H.; Kita, J.; Shiraki, T.; Iso, Y.; Shimoda, M.; Kubota, K. Intraductal papillary neoplasm of the bile duct developing in a patient with primary sclerosing cholangitis: A case report. World J. Gastroenterol. 2014, 20, 15925–15930. [Google Scholar] [CrossRef]
- Kubo, S.; Nakanuma, Y.; Takemura, S.; Sakata, C.; Urata, Y.; Nozawa, A.; Nishioka, T.; Kinoshita, M.; Hamano, G.; Terajima, H.; et al. Case series of 17 patients with cholangiocarcinoma among young adult workers of a printing company in Japan. J. Hepatobiliary Pancreat. Sci. 2014, 21, 479–488. [Google Scholar] [CrossRef]
- Lim, J.H.; Zen, Y.; Jang, K.T.; Kim, Y.K.; Nakanuma, Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: Description of imaging and pathologic aspects. AJR Am. J. Roentgenol. 2011, 197, 1111–1120. [Google Scholar] [CrossRef]
- Watanabe, A.; Suzuki, H.; Kubo, N.; Araki, K.; Kobayashi, T.; Sasaki, S.; Wada, W.; Arai, H.; Sakamoto, K.; Sakurai, S.; et al. An oncocytic variant of intraductal papillary neoplasm of the bile duct that formed a giant hepatic cyst. Rare Tumors 2013, 5, e30. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, Y.; Zen, Y.; Hirano, S.; Tanaka, E.; Takahashi, O.; Yonemori, A.; Doumen, H.; Kawakami, H.; Itoh, T.; Nakanuma, Y.; et al. Intraductal oncocytic papillary neoplasm of the bile duct: The first case of peribiliary gland origin. J. Hepatobiliary Pancreat. Surg. 2009, 16, 869–873. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Sato, Y. Cystic and papillary neoplasm involving peribiliary glands: A biliary counterpart of branch-type intraductal papillary mucinous [corrected] neoplasm? Hepatology 2012, 55, 2040–2041. [Google Scholar] [CrossRef]
- Kang, M.J.; Jang, J.Y.; Lee, K.B.; Han, I.W.; Kim, S.W. Impact of macroscopic morphology, multifocality, and mucin secretion on survival outcome of intraductal papillary neoplasm of the bile duct. J. Gastrointest Surg. 2013, 17, 931–938. [Google Scholar] [CrossRef]
- Aoki, Y.; Mizuma, M.; Hata, T.; Aoki, T.; Omori, Y.; Ono, Y.; Mizukami, Y.; Unno, M.; Furukawa, T. Intraductal papillary neoplasms of the bile duct consist of two distinct types specifically associated with clinicopathological features and molecular phenotypes. J. Pathol. 2020, 251, 38–48. [Google Scholar] [CrossRef]
- Doi, R.; Fukumura, Y.; Lu, R.; Hirabayashi, K.; Kinowaki, Y.; Nakanuma, Y.; Kanai, Y.; Nakahodo, J.; Sasahara, N.; Saito, T.; et al. DNMT1 expression and DNA methylation in intraductal papillary neoplasms of the bile duct. Anticancer Res. 2022, 42, 2893–2902. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Kakuda, Y.; Fukumura, Y.; Sugino, T.; Uesaka, K.; Serizawa, M.; Terada, T.; Ohnishi, Y. The pathologic and genetic characteristics of the intestinal subtype of intraductal papillary neoplasms of the bile duct. Am. J. Surg. Pathol. 2019, 43, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Hatzibougias, D.I.; Fouzas, I.; Bobos, M.; Papanikolaou, V.; Daoudaki, M.; Kotoula, V.; Hytiroglou, P.; Albores-Saavedra, J. Tubular pyloric gland adenoma of the left and right hepatic ducts: Report of a unique case with immunohistochemical and molecular studies. Int. J. Surg. Pathol. 2016, 24, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Kuboki, Y.; Hatori, T.; Yamamoto, M.; Shimizu, K.; Shiratori, K.; Shibata, N.; Shimizu, M.; Furukawa, T. The discrete nature and distinguishing molecular features of pancreatic intraductal tubulopapillary neoplasms and intraductal papillary mucinous neoplasms of the gastric type, pyloric gland variant. J. Pathol. 2013, 231, 335–341. [Google Scholar] [CrossRef]
- Liszka, L.; Pajak, J.; Zielińska-Pajak, E.; Krzych, L.; Gołka, D.; Mrowiec, S.; Lampe, P. Intraductal oncocytic papillary neoplasms of the pancreas and bile ducts: A description of five new cases and review based on a systematic survey of the literature. J. Hepatobiliary Pancreat. Sci. 2010, 17, 246–261. [Google Scholar] [CrossRef]
- Wan, X.S.; Xu, Y.Y.; Qian, J.Y.; Yang, X.B.; Wang, A.Q.; He, L.; Zhao, H.T.; Sang, X.T. Intraductal papillary neoplasm of the bile duct. World J. Gastroenterol. 2013, 19, 8595–8604. [Google Scholar] [CrossRef] [PubMed]
- Onishi, I.; Kitagawa, H.; Harada, K.; Maruzen, S.; Sakai, S.; Makino, I.; Hayashi, H.; Nakagawara, H.; Tajima, H.; Takamura, H.; et al. Intraductal papillary neoplasm of the bile duct accompanying biliary mixed adenoneuroendocrine carcinoma. World J. Gastroenterol. 2013, 19, 3161–3164. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, D.U.; Nam, H.S.; Kang, D.H.; Jang, S.I.; Lee, D.K.; Shin, D.W.; Cho, K.B.; Yang, M.J.; Hwang, J.C.; et al. Comparison of the malignant predictors in intrahepatic and extrahepatic intraductal papillary neoplasm of the bile duct. J. Clin. Med. 2022, 11, 1985. [Google Scholar] [CrossRef]
- Kageyama, Y.; Yamaguchi, R.; Watanabe, S.; Aizu, K.; Sato, F.; Arimoto, A. Intraductal papillary neoplasm of the bile duct with rapidly progressive multicentric recurrence: A case report. Int. J. Surg. Case Rep. 2018, 51, 102–106. [Google Scholar] [CrossRef]
- Nakayama, Y.; Tomino, T.; Ninomiya, M.; Minagawa, R.; Oshiro, Y.; Izumi, T.; Taniguchi, D.; Hirose, K.; Kajiwara, Y.; Minami, K.; et al. Recurrent intraductal papillary neoplasm of the bile duct due to intraductal dissemination: A case report and literature review. Surg. Case Rep. 2021, 7, 238. [Google Scholar] [CrossRef]
- Yokode, M.; Yamashita, Y.; Zen, Y. Biliary intraductal papillary neoplasm with metachronous multiple tumors—True multicentric tumors or intrabiliary dissemination: A case report and review of the literature. Mol. Clin. Oncol. 2017, 6, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Pedica, F.; Heaton, N.; Quaglia, A. Peribiliary glands pathology in a large series of end-stage alcohol-related liver disease. Virchows Arch. 2020, 477, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Harada, K.; Sasaki, M.; Nakanuma, Y. Cystic and micropapillary epithelial changes of peribiliary glands might represent a precursor lesion of biliary epithelial neoplasms. Virchows Arch. 2014, 464, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tanaka, K.; Hirata, A.; Okada, H.; Imai, H.; Shirakami, Y.; Ohnishi, K.; Sugie, S.; Aoki, H.; Hatano, Y.; et al. Inhibition of FGF10-ERK signal activation suppresses intraductal papillary neoplasm of the bile duct and its associated carcinomas. Cell Rep. 2021, 34, 108772. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, S.; Watanabe, M.; Kinoshita, M.; Yamashita, R.; Haeno, H.; Takemura, S.; Tanaka, S.; Marubashi, S.; Totsuka, Y.; Shibata, T.; et al. Multifocal origin of occupational cholangiocarcinoma revealed by comparison of multilesion mutational profiles. Carcinogenesis 2020, 41, 368–376. [Google Scholar] [CrossRef]
- Stendahl, K.; Gilani, S.M.; Basturk, O.; Hui, P.; Sigel, C.; Cai, G. Intraductal papillary neoplasm of the bile duct: Cytomorphologic and molecular features. Cancer Cytopathol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Yang, C.Y.; Huang, W.J.; Tsai, J.H.; Cheng, A.; Chen, C.C.; Hsu, H.P.; Jeng, Y.M. Targeted next-generation sequencing identifies distinct clinicopathologic and molecular entities of intraductal papillary neoplasms of the bile duct. Mod. Pathol. 2019, 32, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Sato, Y. Insulin-like growth factor II mRNA-binding protein 3 (IMP3) is a marker that predicts presence of invasion in papillary biliary tumors. Hum. Pathol. 2017, 62, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Matsubara, T.; Yoneda, N.; Nomoto, K.; Tsuneyama, K.; Sato, Y.; Nakanuma, Y. Overexpression of enhancer of zeste homolog 2 and MUC1 may be related to malignant behaviour in intraductal papillary neoplasm of the bile duct. Histopathology 2013, 62, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Xian, Z.H.; Qin, C.; Cong, W.M. KRAS mutation and immunohistochemical profile in intraductal papillary neoplasm of the intrahepatic bile ducts. Pathol. Res. Pract. 2018, 214, 105–111. [Google Scholar] [CrossRef]
- Tsai, J.H.; Liau, J.Y.; Yuan, C.T.; Cheng, M.L.; Yuan, R.H.; Jeng, Y.M. RNF43 mutation frequently occurs with GNAS mutation and mucin hypersecretion in intraductal papillary neoplasms of the bile duct. Histopathology 2017, 70, 756–765. [Google Scholar] [CrossRef]
- Date, K.; Ohtsuka, T.; Fujimoto, T.; Gotoh, Y.; Nakashima, Y.; Kimura, H.; Matsunaga, T.; Mori, Y.; Mochidome, N.; Miyazaki, T.; et al. GNAS and KRAS mutational analyses of intraductal papillary neoplasms of the pancreas and bile duct developing in the same individual: A case report. Pancreatology 2015, 1, 713–716. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yuan, R.H.; Chen, Y.L.; Liau, J.Y.; Jeng, Y.M. GNAS Is frequently mutated in a specific subgroup of intraductal papillary neoplasms of the bile duct. Am. J. Surg. Pathol. 2013, 37, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, K.; Akita, M.; Ajiki, T.; Fukumoto, T.; Itoh, T.; Zen, Y. Recurrent Mutations in APC and CTNNB1 and Activated Wnt/β-catenin Signaling in Intraductal Papillary Neoplasms of the Bile Duct: A Whole Exome Sequencing Study. Am. J. Surg. Pathol. 2018, 42, 1674–1685. [Google Scholar] [CrossRef]
- Nakahodo, J.; Fukumura, Y.; Saito, T.; Hirabayashi, K.; Doi, R.; Hayashi, T.; Yao, T. Upregulation of follistatin and low apoptotic activity in intraductal oncocytic papillary neoplasm of the pancreatobiliary system. Sci. Rep. 2020, 10, 8179. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Park, K.M.; Lee, S.S.; Yu, E.; Hong, S.M.; Kim, J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J. Hepatol. 2012, 57, 787–793. [Google Scholar]
- Nakanuma, Y.; Kakuda, Y.; Uesaka, K.; Miyata, T.; Yamamoto, Y.; Fukumura, Y.; Sato, Y.; Sasaki, M.; Harada, K.; Takase, M. Characterization of intraductal papillary neoplasm of bile duct with respect to histopathologic similarities to pancreatic intraductal papillary mucinous neoplasm. Hum. Pathol. 2016, 51, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Onoe, S.; Shimoyama, Y.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Nakamura, S.; Nagino, M. Prognostic delineation of papillary cholangiocarcinoma based on the invasive proportion: A single-institution study with 184 patients. Surgery 2014, 155, 280–291. [Google Scholar] [CrossRef]
- Harada, F.; Matsuyama, R.; Mori, R.; Kumamoto, T.; Morioka, D.; Taguri, M.; Yamanaka, S.; Endo, I. Outcomes of surgery for 2010 WHO classification-based intraductal papillary neoplasm of the bile duct: Case-control study of a single Japanese institution’s experience with special attention to mucin expression patterns. Eur. J. Surg. Oncol. 2019, 45, 761–768. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Bowne, W.; Klimstra, D.S.; Ben-Porat, L.; Roggin, K.; Cymes, K.; Fong, Y.; DeMatteo, R.P.; D’Angelica, M.; Koea, J.; et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann. Surg. 2005, 241, 703–712; discussion 712–714. [Google Scholar] [CrossRef]
- Kim, W.J.; Hwang, S.; Lee, Y.J.; Kim, K.H.; Park, K.M.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; et al. Clinicopathological features and long-term outcomes of intraductal papillary neoplasms of the intrahepatic bile duct. J. Gastrointest. Surg. 2016, 20, 1368–1375. [Google Scholar] [CrossRef]
- Luvira, V.; Pugkhem, A.; Bhudhisawasdi, V.; Pairojkul, C.; Sathitkarnmanee, E.; Luvira, V.; Kamsa-Ard, S. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. J. Gastroenterol. Hepatol. 2017, 32, 527–533. [Google Scholar] [CrossRef]
- Onoe, S.; Ebata, T.; Yokoyama, Y.; Igami, T.; Mizuno, T.; Yamaguchi, J.; Watanabe, N.; Otsuka, S.; Nakamura, S.; Shimoyama, Y.; et al. A clinicopathological reappraisal of intraductal papillary neoplasm of the bile duct (IPNB): A continuous spectrum with papillary cholangiocarcinoma in 181 curatively resected cases. HPB 2021, 23, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Igami, T.; Nagino, M.; Oda, K.; Nishio, H.; Ebata, T.; Yokoyama, Y.; Shimoyama, Y. Clinicopathologic study of cholangiocarci noma with superficial spread. Ann. Surg. 2009, 249, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, S.Y.; Kim, H.J.; Lee, S.S.; Hong, G.S.; Byun, J.H.; Hong, S.M.; Lee, M.G. Intraductal papillary neoplasm of the bile duct: Clinical, imaging, and pathologic features. AJR Am. J. Roentgenol. 2018, 211, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, A.; Sumida, Y.; Tamaru, N.; Nakanuma, Y.; Abo, T.; Tanaka, K.; Sawai, T.; Yasutake, T.; Nagayasu, T.; Hayashi, T.; et al. Intraductal papillary neoplasm of the bile duct extending superficially from the intrahepatic to extrahepatic bile duct. J. Gastroenterol. 2006, 41, 495–499. [Google Scholar] [CrossRef]
- Pehlivanoglu, B.; Adsay, V. Intraductal tubulopapillary neoplasms (ITPNs) of the bile ducts: Identity, clinicopathologic characteristics and differential diagnosis of a distinct entity among intraductal tumors. Hum. Pathol. 2022, in press. [Google Scholar] [CrossRef]
- Kuan, L.L.; Dennison, A.R.; Garcea, G. Intraductal tubulopapillary neoplasm of the pancreas and bile duct: A review. Pancreas 2020, 49, 498–502. [Google Scholar] [CrossRef]
- Katabi, N.; Torres, J.; Klimstra, D.S. Intraductal tubular neoplasms of the bile ducts. Am. J. Surg. Pathol. 2012, 36, 1647–1655. [Google Scholar]
- Gross, C.; Engleitner, T.; Lange, S.; Weber, J.; Jesinghaus, M.; Konukiewitz, B.; Muckenhuber, A.; Steiger, K.; Pfarr, N.; Goeppert, B.; et al. Whole exome sequencing of biliary tubulopapillary neoplasms reveals common mutations in chromatin remodeling genes. Cancers 2021, 13, 2742. [Google Scholar]
- Utsumi, Y.; Miyai, K.; Sato, C.; Nishiyama, K.; Murayama, M.; Takeo, H. Polypoid-type adenomyomatous lesion of the cystic duct: Report of a case and review of literature. Int. J. Surg. Pathol. 2022; 10668969221102545, online ahead of print. [Google Scholar] [CrossRef]
- Sugimachi, K.; Mano, Y.; Matsumoto, Y.; Iguchi, T.; Taguchi, K.; Hisano, T.; Sugimoto, R.; Morita, M.; Toh, Y. Adenomyomatous hyperplasia of the extrahepatic bile duct: A systematic review of a rare lesion mimicking bile duct carcinoma. Clin. J. Gastroenterol. 2021, 14, 393–401. [Google Scholar]
- Chandler, P.; Harris, J.; Sherwinter, D.J. Adenomyomatous hyperplasia of distal common bile duct: A case report and review of the literature. Surg. Case Rep. 2018, 2018, rjy204. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Nomura, Y.; Watanabe, H.; Terada, T. Polypoid invasive carcinoma of bile duct: Report of four cases. Clin. J. Gastroenterol. 2022, 15, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Patel, H.; Patel, C. Intrabiliary hepatic metastasis of colorectal carcinoma mimicking primary cholangiocarcinoma: A case report and review of the literature. Case Rep. Pathol. 2016, 2016, 4704781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochidome, N.; Koga, Y.; Ohishi, Y.; Miyazaki, T.; Matsuda, R.; Yamada, Y.; Aishima, S.; Nakamura, M.; Oda, Y. Prognostic implications of the coexisting precursor lesion types in invasive gallbladder cancer. Hum. Pathol. 2021, 114, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Nakanuma, Y.; Nomura, Y.; Watanabe, H.; Terada, T.; Sato, Y.; Kakuda, Y.; Sugino, T.; Ohnishi, Y.; Okamura, Y. Pathological characterization of intracholecystic papillary neoplasm: A recently proposed preinvasive neoplasm of gallbladder. Ann. Diagn. Pathol. 2021, 52, 151723. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Chablé-Montero, F.; González-Romo, M.A.; Ramírez Jaramillo, M.; Henson, D.E. Adenomas of the gallbladder. Morphologic features, expression of gastric and intestinal mucins, and incidence of high-grade dysplasia/carcinoma in situ and invasive carcinoma. Hum. Pathol. 2012, 43, 1506–1513. [Google Scholar] [CrossRef]
- Hazarika, P.; Sharma, M.K. Intracholecystic papillary-tubular neoplasm of gallbladder: A 5-year retrospective pathological study. Indian J. Pathol. Microbiol. 2018, 61, 516–519. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Tuck, M.; McLaren, B.K.; Carrick, K.S.; Henson, D.E. Papillary carcinomas of the gallbladder: Analysis of noninvasive and invasive types. Arch. Pathol. Lab. Med. 2005, 129, 905–909. [Google Scholar] [CrossRef]
- Taskin, O.C.; Basturk, O.; Reid, M.D.; Dursun, N.; Bagci, P.; Saka, B.; Balci, S.; Memis, B.; Bellolio, E.; Araya, J.C.; et al. Gallbladder polyps: Correlation of size and clinicopathologic characteristics based on updated definitions. PLoS ONE 2020, 15, e0237979. [Google Scholar] [CrossRef]
- McGregor, J.C.; Cordiner, J.W. Papilloma of the gallbladder. Br. J. Surg. 1974, 61, 356–358. [Google Scholar]
- Pehlivanoglu, B.; Balci, S.; Basturk, O.; Bagci, P.; Erbarut Seven, I.; Memis, B.; Dursun, N.; Jang, K.T.; Saka, B.; Ohike, N.; et al. Intracholecystic tubular non-mucinous neoplasm (ICTN) of the gallbladder: A clinicopathologically distinct, invasion-resistant entity. Virchows Arch. 2021, 478, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Trisal, M.; Khan, S.; Husain, M.; Ahmad, N.; Hassan, M.J.; Jetley, S. Recently described entity of intracholecystic papillary neoplasm of gallbladder with coexisting xanthogranulomatous cholecystitis: An unusual association. Indian J. Surg. Oncol. 2021, 12, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Otsuka, Y.; Miyata, Y.; Einama, T.; Tsujimoto, H.; Ueno, H.; Ogata, S.; Kishi, Y. Intracholecystic papillary neoplasm arising in a patient with pancreaticobiliary maljunction: A case report. World J. Surg. Oncol. 2020, 18, 292. [Google Scholar] [CrossRef] [PubMed]
- Iseki, M.; Mizuma, M.; Aoki, Y.; Aoki, S.; Hata, T.; Takadate, T.; Kawaguchi, K.; Masuda, K.; Ishida, M.; Ohtsuka, H.; et al. Intracholecystic papillary neoplasm arising in the cystic duct and extending into common bile duct: A case report. Clin. J. Gastroenterol. 2021, 14, 668–677. [Google Scholar] [CrossRef]
- Oishi, K.; Ikeda, M.; Toyota, K.; Mandai, K.; Takahashi, T. Intracholecystic papillary neoplasm localized to the cystic duct: A case report. Case Rep. Gastroenterol. 2022, 16, 66–67. [Google Scholar] [CrossRef]
- Akita, M.; Fujikura, K.; Ajiki, T.; Fukumoto, T.; Otani, K.; Hirose, T.; Tominaga, M.; Itoh, T.; Zen, Y. Intracholecystic papillary neoplasms are distinct from papillary gallbladder cancers: A clinicopathologic and exome-sequencing study. Am. J. Surg. Pathol. 2019, 43, 783–791. [Google Scholar] [CrossRef]
- Logrado, A.; Constantino, J.; Daniel, C.; Pereira, J.; Carvalho, M.T.; Casimiro, C. Low-grade dysplastic intracholecystic papillary neoplasia: A case report. Am. J. Case Rep. 2021, 22, e929788. [Google Scholar] [CrossRef]
- Sciarra, A.; Missiaglia, E.; Trimech, M.; Melloul, E.; Brouland, J.P.; Sempoux, C.; La Rosa, S. Gallbladder mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) arising in intracholecystic papillary neoplasm: Clinicopathologic and molecular analysis of a case and review of the literature. Endocr. Pathol. 2020, 31, 84–93. [Google Scholar] [CrossRef]
- Oh, C.H.; Dong, S.H. Progression to invasive cancer after snare polypectomy of intracholecystic papillary neoplasms during gallbladder stone removal by percutaneous transhepatic choledochoscopy: A case report. BMC Gastroenterol. 2020, 20, 404. [Google Scholar] [CrossRef]
- Kozuka, S.; Tsubone, N.; Yasui, A.; Hachisuka, K. Relation of adenoma to carcinoma in the gallbladder. Cancer 1982, 50, 2226–2234. [Google Scholar] [CrossRef]
- Rowan, D.J.; Pehlivanoglu, B.; Memis, B.; Bagci, P.; Erbarut, I.; Dursun, N.; Jang, K.T.; Sarmiento, J.; Mucientes, F.; Cheng, J.D.; et al. Mural intracholecystic neoplasms arising in adenomyomatous nodules of the gallbladder: An analysis of 19 examples of a clinicopathologically distinct entity. Am. J. Surg. Pathol. 2020, 44, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Muranushi, R.; Saito, H.; Matsumoto, A.; Kato, T.; Tanaka, N.; Nakazato, K.; Morinaga, N.; Shitara, Y.; Ishizaki, M.; Yoshida, T.; et al. A case report of intracholecystic papillary neoplasm of the gallbladder resembling a submucosal tumor. Surg. Case Rep. 2018, 4, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pai, R.K.; Mojtahed, K.; Pai, R.K. Mutations in the RAS/RAF/MAP kinase pathway commonly occur in gallbladder adenomas but are uncommon in gallbladder adenocarcinomas. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 133–140. [Google Scholar] [CrossRef]
- Matthaei, H.; Wu, J.; Dal Molin, M.; Debeljak, M.; Lingohr, P.; Katabi, N.; Klimstra, D.S.; Adsay, N.V.; Eshleman, J.R.; Schulick, R.D.; et al. GNAS codon 201 mutations are uncommon in intraductal papillary neoplasms of the bile duct. HPB 2012, 14, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Berger, Y.; Sullivan, B.J.; Leigh, N.L.; Bekhor, E.Y.; Dhorajiya, P.; Mani, M.; Magge, D.R.; Cha, D.E.; Sarpel, U.; Hiotis, S.P.; et al. Gallbladder cancer: A single-institution 10-year experience-analysis of adenocarcinoma subtypes and tumors arising from intracholecystic papillary neoplasms. Ann. Surg. Oncol. 2022, 29, 5167–5175. [Google Scholar] [CrossRef]
- He, C.; Fukumura, Y.; Toriyama, A.; Ogura, K.; Sasahara, N.; Mitani, K.; Yao, T. Pyloric gland adenoma (PGA) of the gallbladder: A unique and distinct tumor from PGAs of the stomach, duodenum, and pancreas. Am. J. Surg. Pathol. 2018, 42, 1237–1245. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Sugino, T.; Nomura, K.; Terada, T.; Sato, Y.; Ohnishi, Y. Pathological features of pyloric gland adenoma of the gallbladder in comparison with gastric subtype of intracholecystic papillary neoplasm. Ann. Diagn. Pathol. 2022, 56, 151879. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Sugino, T.; Nomura, Y.; Watanabe, H.; Terada, T. Polypoid invasive carcinoma of the gallbladder-Another challenging polypoid neoplasm. J. Hepatobiliary Pancreat. Sci. 2022, 29, 531–539. [Google Scholar] [CrossRef]




| Intestinal Subtype | Gastric Subtype | PB Subtype | Oncocytic Subtype | |
|---|---|---|---|---|
| Histologic features | Columnar epithelia with single-layered or pseudostratified cigar-shaped nuclei and with basophilic, amphophilic cytoplasm with apical vesicular mucin. Tubular, papillary/villous and serrated structures. Some show villous/papillary pattern, while the other showed tubular or tubule-villous pattern. | Regional growth of papillary and tubular (glandular) neoplastic epithelia resembling gastric foveolar epithelia and pyloric glands. Proportion of these two components is variable: some cases showed predominantly foveolar pattern or pyloric gland pattern, while the other showed equal amount of both components | Single-layered, small to medium cuboidal/low-columnar, neoplastic- epithelia. Centrally or basally located, nuclei and slightly acidophilic cytoplasm. Epithelia cell and nuclei resembling simple epithelia of bile or pancreatic duct. Many fine papillary structures with many ramifying branches of thin fibrovascular stalks. | Single- to multi-layered medium-sized cuboidal to low-columnar epithelia with eosinophilic. granular cytoplasm. Frequent secondary lumina formation. Arborized papillary and/or cribriform formations. Thin or edematous or inflammatory stroma |
| Immunohisto- chemical features | Frequently positive for CDX2 and CK20. Goblet cells positive for MUC2. | Foveolar components positive for MUC5AC, and pyloric glands positive for MUC6. | Positive for CK7, S100P and MUC1. | Positive for gastric-type mucin (MUC5AC and MUC6) and for HepPar-1 |
| Anatomical Locations | Precursors |
|---|---|
| Intrahepatic large bile duct Perihilar bile duct Distal bile duct | BilIN (low-grade and high-grade) IPNB MCN |
| Gallbladder | BilIN (low-grade and high-grade) |
| ICPN | |
| MCN | |
| Pyloric gland adenoma | |
| Pancreas | PanIN (low-grade and high-grade) |
| IPMN | |
| MCN | |
| IOPN | |
| ITPN | |
| Ampulla | Intestinal adenoma (low-grade and high-grade) |
| IAPN | |
| Flat intraepithelial neoplasm |
| Low-Grade | High-Grade | |
|---|---|---|
| Histology | Flat/pseudopapillary/micropapillary | Flat/pseudopapillary/micropapillary/thickened |
| appearance | mucosa appearance | |
| Hyperchromatic nuclei | Hyperchromatic and irregular nuclei | |
| Increased N/C ratio | Pleomorphic, bizarre nuclei, increased N/C ratio | |
| Nuclear stratification | Complex nuclear stratification | |
| Preserved nuclear polarity | Loss of nuclear polarity | |
| Biliary mucosa involvement | Relatively small foci or areas | Relatively extensive area |
| Involvement of peribiliary glands/RAS | Infrequent | Frequent |
| Ki-67 index | Mildly to moderately increased | Markedly increased |
| Immunostainings | ||
| S100P | Mild to moderately increased | Diffuse and strongly positive |
| P53 | Usually negative | Frequently positive |
| P16 | Relatively preserved | Decreased |
| Intraepithelial Lateral (Superficial) Spreading around High-Grade BilIN, IPNB and ICPN | Glandular Involvement Beneath High-Grade BilIN, IPNB and ICPN | |
|---|---|---|
| Affected tissues | Surrounding mucosa (non-neoplastic lining epithelia and mucosal glands) of the bile duct and gallbladder | Bile ducts: Peribiliary glands and their conduits in the duct wall and periductal tissue |
| Gallbladder: RAS and non-neoplastic tubules in the wall and subserosa | ||
| Characteristic growth patterns | Neoplastic epithelia show abrupt transition against non-neoplastic epithelia | Mixture of neoplastic epithelia and non-neoplastic epithelia within peribiliary glands and their conduits or RAS and non-neoplastic tubules |
| Neoplastic epithelia show replacing spread in the mucosa for considerable areas and length (occasionally involving more than one anatomical segments of biliary tract) | Continuous and discontinuous involvement of peribiliary glands and their conduits or tubules and RAS from neoplastic epithelia of luminal surface | |
| Spreading neoplastic epithelia show the same cell lineage of the high-grade BilIN, IPNB or ICPN | Involving neoplastic epithelia show the same cell lineage of the high-grade BilIN, IPNB or ICPN | |
| Not associated with desmoplastic reaction | Not associated with desmoplastic reaction | |
| Significance | Growth and extension of high-grade BilIN and IPNB and ICPN | Growth and extension of high-grade BilIN and IPNB and ICPN |
| Surgical margin at operation | Diagnostic pitfall for invasive growth |
| Type 1 | Type 2 | |
|---|---|---|
| Findings for classification | ||
| Prototypic structures of four subtypes | Retained and regular | Variably lost and irregular |
| Gastric, pancreatobiliary subtypes | Similar to prototypes of IPMN * of pancreas | Variably different from prototypes of IPMN * of pancreas |
| Oncocytic subtype | Similar to IOPN ** of pancreas | Variably different from prototype of IOPN ** of pancreas |
| Intestinal subtype | Similar to intestinal adenoma of ampulla | Variable different from prototype of intestinal adenoma of ampulla |
| Cellular and nuclear atypia | Low-grade or low- and high-grade | High-grade including overt malignancy |
| Cribriform and solid growth | None | Not infrequent |
| Necrosis | None | Not infrequent |
| Other features | ||
| Location intrahepatic | 58–68% | 14–27% |
| extrahepatic | 32–35% | 48–64% |
| both | 7% | 22% |
| Mucus hypersecretion | 29–86% | 12–21% |
| Invasion | 6–50% | 60–94% |
| Solitary Type | Conglomerated Type | |
|---|---|---|
| Gross features | Pedunculated, polypoid | Sessile, multiple or coalescent, excrescences |
| Border | Well demarcated from the surrounding mucosa | Continuous with surrounding granular or rough mucosa |
| Involvement | One to more areas * | Localized to one area |
| Background mucosa lesion | No or very limited intraepithelial neoplasm, dysplasia | Wide spreading of micropapillary and flat intraepithelial neoplasm, dysplasia |
| Stromal Invasion | Infrequent | Frequent |
| Incidence | About 25% of ICPN | About 75% of ICPN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanuma, Y.; Kakuda, Y.; Sugino, T.; Sato, Y.; Fukumura, Y. Pathologies of Precursor Lesions of Biliary Tract Carcinoma. Cancers 2022, 14, 5358. https://doi.org/10.3390/cancers14215358
Nakanuma Y, Kakuda Y, Sugino T, Sato Y, Fukumura Y. Pathologies of Precursor Lesions of Biliary Tract Carcinoma. Cancers. 2022; 14(21):5358. https://doi.org/10.3390/cancers14215358
Chicago/Turabian StyleNakanuma, Yasuni, Yuko Kakuda, Takashi Sugino, Yasunori Sato, and Yuki Fukumura. 2022. "Pathologies of Precursor Lesions of Biliary Tract Carcinoma" Cancers 14, no. 21: 5358. https://doi.org/10.3390/cancers14215358