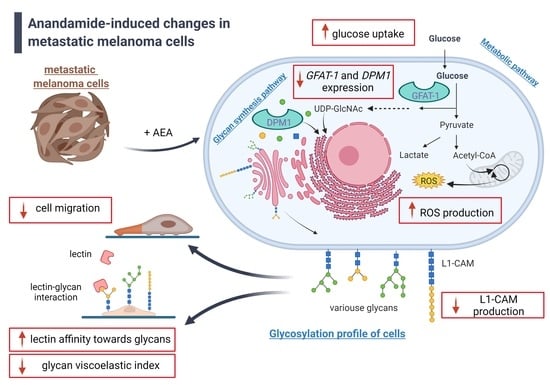
Anandamide-Modulated Changes in Metabolism, Glycosylation Profile and Migration of Metastatic Melanoma Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Anandamide Treatment
2.3. MTT Assay
2.4. Crystal Violet (CV) Assay
2.5. FDA/PI Assay
2.6. Glucose Uptake Assay
2.7. Reactive Oxygen Species (ROS) Quantification Test
2.8. RNA Isolation Followed by the Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. ELISA In Situ
2.10. QCM-D Lectin–Glycan Measurements
2.11. Lectin-ELISA Assay
2.12. Scratch Assay
2.13. Elasticity of Single Cells
2.14. Actin Filaments and Cell Nuclei Visualization
2.15. Statistical Analysis
3. Results
3.1. Melanoma Cell Viability after Anandamide Treatment
3.2. Influence of AEA on Melanoma Cell Metabolism
3.3. Changes in Glycosyltranferases Expression in the AEA-Treated Melanoma Cells
3.4. AEA-Induced Changes in L1-CAM Production by Melanoma Cells
3.5. Biophysical Measurements of Changes in the Glycosylation Profile of AEA-Treated Melanoma Cells
3.6. Anandamide-Induced Changes to Melanoma Cell Migration
3.7. Unchanged Elastic Properties of Melanoma Cells Treated with AEA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer, J.E.; Swetter, S.M.; Fu, T.; Geller, A.C. Screening, Early Detection, Education, and Trends for Melanoma: Current Status (2007–2013) and Future Directions. J. Am. Acad. Dermatol. 2014, 71, 599.e1–599.e12. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-Induced Skin Damage. Toxicology 2003, 189, 21–39. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-Tyrosine and L-Dihydroxyphenylalanine as Hormone-like Regulators of Melanocyte Functions: L-Tyrosine and L-DOPA as Bioregulators. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagna, R.; Pozzi, V.; Sartini, D.; Salvolini, E.; Brisigotti, V.; Molinelli, E.; Campanati, A.; Offidani, A.; Emanuelli, M. Beyond Nicotinamide Metabolism: Potential Role of Nicotinamide N-Methyltransferase as a Biomarker in Skin Cancers. Cancers 2021, 13, 4943. [Google Scholar] [CrossRef] [PubMed]
- McDermott, N.C.; Hayes, D.P.; al-Sader, M.H.; Hogan, J.M.; Walsh, C.B.; Kay, E.W.; Leader, M.B. Identification of Vertical Growth Phase in Malignant Melanoma. A Study of Interobserver Agreement. Am. J. Clin. Pathol. 1998, 110, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Shih, D.T.; Meier, F.E.; Van Belle, P.; Hsu, J.Y.; Elder, D.E.; Buck, C.A.; Herlyn, M. Adenoviral Gene Transfer of Beta3 Integrin Subunit Induces Conversion from Radial to Vertical Growth Phase in Primary Human Melanoma. Am. J. Pathol. 1998, 153, 1435–1442. [Google Scholar] [CrossRef]
- Elder, D.E. Melanoma Progression. Pathology 2016, 48, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Akkooi, A.C.J.; Hieken, T.J.; Burton, E.M.; Ariyan, C.; Ascierto, P.A.; Asero, S.V.M.A.; Blank, C.U.; Block, M.S.; Boland, G.M.; Caraco, C.; et al. Neoadjuvant Systemic Therapy (NAST) in Patients with Melanoma: Surgical Considerations by the International Neoadjuvant Melanoma Consortium (INMC). Ann. Surg. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Krayem, M.; Ghanem, G.E.; Van Gestel, D. Recent Advances in Radiosensitivity Determinants in Melanoma. Curr. Opin. Oncol. 2022, 34, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Trakatelli, M.; de Vries, E.; Katsanos, G.; Tzachanis, D.; Eggermont, A. Cutaneous Malignant Melanoma. In Managing Skin Cancer; Springer: Berlin/Heidelberg, Germany, 2010; pp. 79–110. ISBN 978-3-540-79347-2. [Google Scholar]
- Aris, M.; Barrio, M.M. Combining Immunotherapy with Oncogene-Targeted Therapy: A New Road for Melanoma Treatment. Front. Immunol. 2015, 6, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Liu, W.; Gotlieb, V. The Rapidly Evolving Therapies for Advanced Melanoma—Towards Immunotherapy, Molecular Targeted Therapy, and Beyond. Crit. Rev. Oncol. Hematol. 2016, 99, 91–99. [Google Scholar] [CrossRef]
- Lugowska, I.; Teterycz, P.; Rutkowski, P. Immunotherapy of Melanoma. Contemp. Oncol. 2018, 2018, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Back, M.F. Cutaneous Malignant Melanoma. In Radiation Oncology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 513–527. ISBN 978-3-540-77385-6. [Google Scholar]
- Stanisz, H.; Vogt, T.; Rass, K. Chemotherapy for Melanoma. In Diagnostic and Prognostic Biomarkers and Therapeutic Targets in Melanoma; Humana Press: Totowa, NJ, USA, 2012; pp. 247–263. ISBN 978-1-60761-433-3. [Google Scholar]
- Wolf, J.A.; Moreau, J.F.; Akilov, O.; Patton, T.; English, J.C.; Ho, J.; Ferris, L.K. Diagnostic Inaccuracy of Smartphone Applications for Melanoma Detection. JAMA Dermatol. 2013, 149, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campagna, R.; Bacchetti, T.; Salvolini, E.; Pozzi, V.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Ferretti, G.; Offidani, A.; et al. Paraoxonase-2 Silencing Enhances Sensitivity of A375 Melanoma Cells to Treatment with Cisplatin. Antioxidants 2020, 9, 1238. [Google Scholar] [CrossRef] [PubMed]
- Pokrywka, M.; Lityńska, A. Celując w czerniaka. Target. Melanoma 2012, 39, 3–23. [Google Scholar]
- Kulms, D.; Meier, F. In Vitro Models of Melanoma. In Skin Tissue Models for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 57–75. ISBN 978-0-12-810545-0. [Google Scholar]
- Campagna, R.; Salvolini, E.; Pompei, V.; Pozzi, V.; Salvucci, A.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Offidani, A.; et al. Nicotinamide N-methyltransferase Gene Silencing Enhances Chemosensitivity of Melanoma Cell Lines. Pigment Cell Melanoma Res. 2021, 34, 1039–1048. [Google Scholar] [CrossRef]
- Yang, K.; Oak, A.S.W.; Slominski, R.M.; Brożyna, A.A.; Slominski, A.T. Current Molecular Markers of Melanoma and Treatment Targets. Int. J. Mol. Sci. 2020, 21, 3535. [Google Scholar] [CrossRef]
- Albertini, M.R.; Longley, B.J.; Harari, P.M.; Reintgen, D. Cutaneous Melanoma. In Oncology; Springer: New York, NY, USA, 2006; pp. 1082–1101. ISBN 978-0-387-31056-5. [Google Scholar]
- Sobiepanek, A.; Paone, A.; Cutruzzolà, F.; Kobiela, T. Biophysical Characterization of Melanoma Cell Phenotype Markers during Metastatic Progression. Eur. Biophys. J. 2021, 50, 523–542. [Google Scholar] [CrossRef]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casadó, V.; Canela, E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharmacol. 2018, 10, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, J.; Hohmann, A.G. The Endocannabinoid System and Cancer: Therapeutic Implication: Cannabinoids and Cancer. Br. J. Pharmacol. 2011, 163, 1447–1463. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.-C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the Endocannabinoid System as a Potential Anticancer Strategy. Front. Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, I.; Cascio, M.G.; Rotondo, D.; Pertwee, R.G.; Heys, S.D.; Wahle, K.W.J. Cannabinoids and Omega-3/6 Endocannabinoids as Cell Death and Anticancer Modulators. Prog. Lipid Res. 2013, 52, 80–109. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Biogenic Amines in the Skin. In Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin’s Neuroendocrine System; Advances in Anatomy, Embryology and Cell Biology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 212, pp. 7–26. ISBN 978-3-642-19682-9. [Google Scholar]
- Velasco, G.; Sánchez, C.; Guzmán, M. Anticancer Mechanisms of Cannabinoids. Curr. Oncol. 2016, 23, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adinolfi, B.; Romanini, A.; Vanni, A.; Martinotti, E.; Chicca, A.; Fogli, S.; Nieri, P. Anticancer Activity of Anandamide in Human Cutaneous Melanoma Cells. Eur. J. Pharmacol. 2013, 718, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Picardi, P.; Ciaglia, E.; Proto, M.; Pisanti, S. Anandamide Inhibits Breast Tumor-Induced Angiogenesis. Transl. Med. UniSa 2014, 10, 8–12. [Google Scholar] [PubMed]
- Laezza, C.; D’Alessandro, A.; Paladino, S.; Maria Malfitano, A.; Chiara Proto, M.; Gazzerro, P.; Pisanti, S.; Santoro, A.; Ciaglia, E.; Bifulco, M. Anandamide Inhibits the Wnt/β-Catenin Signalling Pathway in Human Breast Cancer MDA MB 231 Cells. Eur. J. Cancer 2012, 48, 3112–3122. [Google Scholar] [CrossRef]
- Sobiepanek, A.; Kowalska, P.D.; Szota, M.; Grzywa, T.M.; Nowak, J.; Włodarski, P.K.; Galus, R.; Jachimska, B.; Kobiela, T. Novel Diagnostic and Prognostic Factors for the Advanced Melanoma Based on the Glycosylation-Related Changes Studied by Biophysical Profiling Methods. Biosens. Bioelectron. 2022, 203, 114046. [Google Scholar] [CrossRef] [PubMed]
- Kozon, D.; Mierzejewska, J.; Kobiela, T.; Grochowska, A.; Dudnyk, K.; Głogowska, A.; Sobiepanek, A.; Kuźmińska, A.; Ciach, T.; Augustynowicz-Kopeć, E.; et al. Amphiphilic Polymethyloxazoline–Polyethyleneimine Copolymers: Interaction with Lipid Bilayer and Antibacterial Properties. Macromol. Biosci. 2019, 19, 1900254. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sobiepanek, A.; Milner-Krawczyk, M.; Lekka, M.; Kobiela, T. AFM and QCM-D as Tools for the Distinction of Melanoma Cells with a Different Metastatic Potential. Biosens. Bioelectron. 2017, 93, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sobiepanek, A.; Kobiela, T. Studying the Skin Cells Viscoelastic Changes Using QCM-D Measurements. In Bioengineering Technologies. Methods in Molecular Biology; Rasooly, A., Baker, H., Ossandon, H., Eds.; Humana Press: Totowa, NJ, USA, 2022; Volume 2393, pp. 535–558. ISBN 978-1-07-161803-5. [Google Scholar]
- Kobiela, T.; Lelen-Kaminska, K.; Stepulak, M.; Lekka, M.; Malejczyk, M.; Arct, J.; Majewski, S. The Influence of Surfactants and Hydrolyzed Proteins on Keratinocytes Viability and Elasticity. Ski. Res. Technol. 2013, 19, e200–e208. [Google Scholar] [CrossRef]
- Sobiepanek, A.; Milner-Krawczyk, M.; Bobecka-Wesołowska, K.; Kobiela, T. The Effect of Delphinidin on the Mechanical Properties of Keratinocytes Exposed to UVB Radiation. J. Photochem. Photobiol. B Biol. 2016, 164, 264–270. [Google Scholar] [CrossRef]
- Evrard, Y.A.; Newton, D.; Das, B.; Alcoser, S.Y.; Arthur, K.; Baldwin, M.; Bonomi, C.; Borgel, S.; Carter, J.; Chase, T.; et al. Comparison of PDX, PDC, and PDOrg Models from the National Cancer Institute’s Patient-Derived Models Repository (PDMR). Cancer Res. 2019, 79, 4524. [Google Scholar] [CrossRef]
- Bleijs, M.; Wetering, M.; Clevers, H.; Drost, J. Xenograft and Organoid Model Systems in Cancer Research. EMBO J. 2019, 38, e101654. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Elder, D.E.; Herlyn, M. Melanoma: The Wistar Melanoma (WM) Cell Lines. In Human Cell Culture: Cancer Cell Lines Part 1; Masters, J.R.W., Palsson, B., Eds.; Human Cell Culture; Springer: Dordrecht, The Netherlands, 1999; pp. 259–274. ISBN 978-0-306-46872-8. [Google Scholar]
- Ng, S.; Gisonni-Lex, L.; Azizi, A. New Approaches for Characterization of the Genetic Stability of Vaccine Cell Lines. Hum. Vaccines Immunother. 2017, 13, 1669–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Sun, L.; Liu, M.; Mao, Y. Patient-Derived Organoids: A Promising Model for Personalized Cancer Treatment. Gastroenterol. Rep. 2018, 6, 243–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, R.; Conde, J.; Scotece, M.; López, V.; Lago, F.; Gómez Reino, J.J.; Gualillo, O. Endogenous Cannabinoid Anandamide Impairs Cell Growth and Induces Apoptosis in Chondrocytes: Anandamide Impairs Cell Growth. J. Orthop. Res. 2014, 32, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Sarker, K.P.; Obara, S.; Nakata, M.; Kitajima, I.; Maruyama, I. Anandamide Induces Apoptosis of PC-12 Cells: Involvement of Superoxide and Caspase-3. FEBS Lett. 2000, 472, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Bilmin, K.; Kopczyńska, B.; Grieb, P. Original Article Influence of Serum and Albumin on the in Vitro Anandamide Cytotoxicity toward C6 Glioma Cells Assessed by the MTT Cell Viability Assay: Implications for the Methodology of the MTT Tests. Folia Neuropathol. 2013, 1, 44–50. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Davis, J.B.; Di Marzo, V. Palmitoylethanolamide Enhances Anandamide Stimulation of Human Vanilloid VR1 Receptors. FEBS Lett. 2001, 506, 253–256. [Google Scholar] [CrossRef]
- Fischer, G.M.; Vashisht Gopal, Y.N.; McQuade, J.L.; Peng, W.; DeBerardinis, R.J.; Davies, M.A. Metabolic Strategies of Melanoma Cells: Mechanisms, Interactions with the Tumor Microenvironment, and Therapeutic Implications. Pigment Cell Melanoma Res. 2018, 31, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.; Sell, H.; Taube, A.; Koenen, M.; Platzbecker, B.; Cramer, A.; Horrighs, A.; Lehtonen, M.; Tennagels, N.; Eckel, J. Cannabinoid Type 1 Receptors in Human Skeletal Muscle Cells Participate in the Negative Crosstalk between Fat and Muscle. Diabetologia 2009, 52, 664–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-W.; Kim, J.-E.; Oh, S.-M.; Cha, W.-J.; Hah, J.-H.; Sung, M.-W. Anticancer Effects of Anandamide on Head and Neck Squamous Cell Carcinoma Cells via the Production of Receptor-Independent Reactive Oxygen Species: Anandamide and HNSCC Cell Death. Head Neck 2015, 37, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- DeMorrow, S.; Francis, H.; Gaudio, E.; Ueno, Y.; Venter, J.; Onori, P.; Franchitto, A.; Vaculin, B.; Vaculin, S.; Alpini, G. Anandamide Inhibits Cholangiocyte Hyperplastic Proliferation via Activation of Thioredoxin 1/Redox Factor 1 and AP-1 Activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G506–G519. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos dos Santos, A.; Oliveira, I.A.; Lucena, M.C.; Mantuano, N.R.; Whelan, S.A.; Dias, W.B.; Todeschini, A.R. Biosynthetic Machinery Involved in Aberrant Glycosylation: Promising Targets for Developing of Drugs Against Cancer. Front. Oncol. 2015, 5, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, P.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017. [Google Scholar]
- Schnaar, R.L.; Kinoshita, T. Glycosphingolipids. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017. [Google Scholar]
- Gélinas, R.; Mailleux, F.; Dontaine, J.; Bultot, L.; Demeulder, B.; Ginion, A.; Daskalopoulos, E.P.; Esfahani, H.; Dubois-Deruy, E.; Lauzier, B.; et al. AMPK Activation Counteracts Cardiac Hypertrophy by Reducing O-GlcNAcylation. Nat. Commun. 2018, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aebi, M. Hennet Congenital Disorders of Glycosylation: Genetic Model Systems Lead the Way. Trends Cell Biol. 2001, 11, 136–141. [Google Scholar] [CrossRef]
- Kinoshita, T.; Murakami, Y.; Morita, Y.S. Diseases Associated with GPI Anchors. In Comprehensive Glycoscience; Elsevier: Amsterdam, The Netherlands, 2007; pp. 393–419. ISBN 978-0-444-51967-2. [Google Scholar]
- Eldai, H.; Periyasamy, S.; Al Qarni, S.; Al Rodayyan, M.; Muhammed Mustafa, S.; Deeb, A.; Al Sheikh, E.; Afzal Khan, M.; Johani, M.; Yousef, Z.; et al. Novel Genes Associated with Colorectal Cancer Are Revealed by High Resolution Cytogenetic Analysis in a Patient Specific Manner. PLoS ONE 2013, 8, e76251. [Google Scholar] [CrossRef]
- Banerjee, D.K.; Zhang, Z.; Baksi, K.; Serrano-Negrón, J.E. Dolichol Phosphate Mannose Synthase: A Glycosyltransferase with Unity in Molecular Diversities. Glycoconj. J. 2017, 34, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Lucena, M.C.; Carvalho-Cruz, P.; Donadio, J.L.; Oliveira, I.A.; de Queiroz, R.M.; Marinho-Carvalho, M.M.; Sola-Penna, M.; de Paula, I.F.; Gondim, K.C.; McComb, M.E.; et al. Epithelial Mesenchymal Transition Induces Aberrant Glycosylation through Hexosamine Biosynthetic Pathway Activation. J. Biol. Chem. 2016, 291, 12917–12929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho-cruz, P.; Alisson-Silva, F.; Todeschini, A.R.; Dias, W.B. Cellular Glycosylation Senses Metabolic Changes and Modulates Cell Plasticity during Epithelial to Mesenchymal Transition: Cellular Glycosylation and Changes during EMT. Dev. Dyn. 2018, 247, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Szymura, S.J.; Zaemes, J.P.; Allison, D.F.; Clift, S.H.; D’Innocenzi, J.M.; Gray, L.G.; McKenna, B.D.; Morris, B.B.; Bekiranov, S.; LeGallo, R.D.; et al. NF-ΚB Upregulates Glutamine-Fructose-6-Phosphate Transaminase 2 to Promote Migration in Non-Small Cell Lung Cancer. Cell Commun. Signal. 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, D.K. N-Glycans in Cell Survival and Death: Cross-Talk between Glycosyltransferases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 1338–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Zhu, M.; Qin, Y.; Zhong, Y.; Yan, H.; Wang, Q.; Bian, H.; Li, Z. Analysis of Glycan-Related Genes Expression and Glycan Profiles in Mice with Liver Fibrosis. J. Proteome Res. 2012, 11, 5277–5285. [Google Scholar] [CrossRef] [PubMed]
- Milde-Langosch, K.; Karn, T.; Schmidt, M.; zu Eulenburg, C.; Oliveira-Ferrer, L.; Wirtz, R.M.; Schumacher, U.; Witzel, I.; Schütze, D.; Müller, V. Prognostic Relevance of Glycosylation-Associated Genes in Breast Cancer. Breast Cancer Res. Treat. 2014, 145, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ochwat, D.; Hoja-Łukowicz, D.; Lityńska, A. N-Glycoproteins Bearing B1-6 Branched Oligosaccharides from the A375 Human Melanoma Cell Line Analysed by Tandem Mass Spectrometry. Melanoma Res. 2004, 14, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Hoja-Łukowicz, D.; Link-Lenczowski, P.; Carpentieri, A.; Amoresano, A.; Pocheć, E.; Artemenko, K.A.; Bergquist, J.; Lityńska, A. L1CAM from Human Melanoma Carries a Novel Type of N-Glycan with Galβ1-4Galβ1- Motif. Involvement of N-Linked Glycans in Migratory and Invasive Behaviour of Melanoma Cells. Glycoconj. J. 2013, 30, 205–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, A.-K.; Putscher, A.; Samatov, T.R.; Suling, A.; Galatenko, V.V.; Shkurnikov, M.Y.; Knyazev, E.N.; Tonevitsky, A.G.; Haalck, T.; Lange, T.; et al. Knockdown of L1CAM Significantly Reduces Metastasis in a Xenograft Model of Human Melanoma: L1CAM Is a Potential Target for Anti-Melanoma Therapy. PLoS ONE 2018, 13, e0192525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, M.C. Quartz Crystal Microbalance with Dissipation Monitoring: Enabling Real-Time Characterization of Biological Materials and Their Interactions. J. Biomol. Tech. 2008, 19, 151–158. [Google Scholar] [PubMed]
- Senkara-Barwijuk, E.; Kobiela, T.; Lebed, K.; Lekka, M. Reaction Pathway and Free Energy Profile Determined for Specific Recognition of Oligosaccharide Moiety of Carboxypeptidase Y. Biosens. Bioelectron. 2012, 36, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ścieżyńska, A.; Sobiepanek, A.; Kowalska, P.D.; Soszyńska, M.; Łuszczyński, K.; Grzywa, T.M.; Krześniak, N.; Góźdź, A.; Włodarski, P.K.; Galus, R.; et al. A Novel and Effective Method for Human Primary Skin Melanocytes and Metastatic Melanoma Cell Isolation. Cancers 2021, 13, 6244. [Google Scholar] [CrossRef] [PubMed]
- Potapenko, I.O.; Haakensen, V.D.; Lüders, T.; Helland, Å.; Bukholm, I.; Sørlie, T.; Kristensen, V.N.; Lingjaerde, O.C.; Børresen-Dale, A.-L. Glycan Gene Expression Signatures in Normal and Malignant Breast Tissue; Possible Role in Diagnosis and Progression. Mol. Oncol. 2010, 4, 98–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciołczyk-Wierzbicka, D.; Amoresano, A.; Casbarra, A.; Hoja-Łukowicz, D.; Lityńska, A.; Laidler, P. The Structure of the Oligosaccharides of N-Cadherin from Human Melanoma Cell Lines. Glycoconj. J. 2004, 20, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Przybyło, M.; Martuszewska, D.; Pocheć, E.; Hoja-Łukowicz, D.; Lityńska, A. Identification of Proteins Bearing Β1–6 Branched N-Glycans in Human Melanoma Cell Lines from Different Progression Stages by Tandem Mass Spectrometry Analysis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2007, 1770, 1427–1435. [Google Scholar] [CrossRef]
- Grimaldi, C.; Pisanti, S.; Laezza, C.; Malfitano, A.M.; Santoro, A.; Vitale, M.; Caruso, M.G.; Notarnicola, M.; Iacuzzo, I.; Portella, G.; et al. Anandamide Inhibits Adhesion and Migration of Breast Cancer Cells. Exp. Cell Res. 2006, 312, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Anandamide Is an Endogenous Inhibitor for the Migration of Tumor Cells and T Lymphocytes. Cancer Immunol. Immunother. 2004, 53, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Carpi, S.; Fogli, S.; Polini, B.; Montagnani, V.; Podestà, A.; Breschi, M.C.; Romanini, A.; Stecca, B.; Nieri, P. Tumor-Promoting Effects of Cannabinoid Receptor Type 1 in Human Melanoma Cells. Toxicol. Vitr. 2017, 40, 272–279. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Nabissi, M.; Santoni, G.; Ligresti, A. Actions and Regulation of Ionotropic Cannabinoid Receptors. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 249–289. ISBN 978-0-12-811232-8. [Google Scholar]
- Hohmann, T.; Grabiec, U.; Ghadban, C.; Feese, K.; Dehghani, F. The Influence of Biomechanical Properties and Cannabinoids on Tumor Invasion. Cell Adhes. Migr. 2017, 11, 54–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Cell Type/Cell Line | Sample | KD [nM] | KD Ratio |
|---|---|---|---|
| RGP site—WM35 | CTR | 1.80 | 1.8 |
| AEA | 3.15 | ||
| VGP site—WM115 | CTR | 0.95 | 1.5 |
| AEA | 1.43 | ||
| Metastasis to the lymph node—WM266-4 | CTR | 0.23 | 3.1 |
| AEA | 0.72 | ||
| Solid tumor metastasis—A375-P | CTR | 0.36 | 4.6 |
| AEA | 1.66 |
| Cell Type/Cell Line | Sample | b/a Relation | b/a Ratio |
|---|---|---|---|
| RGP site—WM35 | CTR | 20.7 | 1.0 |
| AEA | 21.1 | ||
| VGP site—WM115 | CTR | 5.6 | 1.3 |
| AEA | 7.0 | ||
| Metastasis to the lymph node—WM266-4 | CTR | 5.3 | 3.0 |
| AEA | 16.0 | ||
| Solid tumor metastasis—A375-P | CTR | 4.5 | 4.1 |
| AEA | 18.6 |
| Cell Type/Cell Line | Sample | gVI | gVI Ratio |
|---|---|---|---|
| RGP site—WM35 | CTR | 0.229 | 1.1 |
| AEA | 0.246 | ||
| VGP site—WM115 | CTR | 0.274 | 1.2 |
| AEA | 0.317 | ||
| Metastasis to the lymph node—WM266-4 | CTR | 0.370 | 0.5 |
| AEA | 0.202 | ||
| Solid tumor metastasis—A375-P | CTR | 0.514 | 0.6 |
| AEA | 0.318 |
| Cell Type/Cell Line | Sample | E [kPa] | E Ratio |
|---|---|---|---|
| RGP site—WM35 | CTR | 3.22 ± 0.86 | 1.08 |
| AEA | 3.47 ± 0.80 | ||
| VGP site—WM115 | CTR | 2.29 ± 0.64 | 1.06 |
| AEA | 2.44 ± 0.64 | ||
| Metastasis to the lymph node—WM266-4 | CTR | 2.14 ± 0.39 | 1.00 |
| AEA | 2.14 ± 0.32 | ||
| Solid tumor metastasis—A375-P | CTR | 2.45 ± 0.49 | 1.14 |
| AEA | 2.80 ± 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobiepanek, A.; Milner-Krawczyk, M.; Musolf, P.; Starecki, T.; Kobiela, T. Anandamide-Modulated Changes in Metabolism, Glycosylation Profile and Migration of Metastatic Melanoma Cells. Cancers 2022, 14, 1419. https://doi.org/10.3390/cancers14061419
Sobiepanek A, Milner-Krawczyk M, Musolf P, Starecki T, Kobiela T. Anandamide-Modulated Changes in Metabolism, Glycosylation Profile and Migration of Metastatic Melanoma Cells. Cancers. 2022; 14(6):1419. https://doi.org/10.3390/cancers14061419
Chicago/Turabian StyleSobiepanek, Anna, Małgorzata Milner-Krawczyk, Paulina Musolf, Tomasz Starecki, and Tomasz Kobiela. 2022. "Anandamide-Modulated Changes in Metabolism, Glycosylation Profile and Migration of Metastatic Melanoma Cells" Cancers 14, no. 6: 1419. https://doi.org/10.3390/cancers14061419