Cancer Therapy Targeting CD47/SIRPα
Abstract
:Simple Summary
Abstract
1. Introduction
2. Innate and Adaptive Immune Systems and Cancer
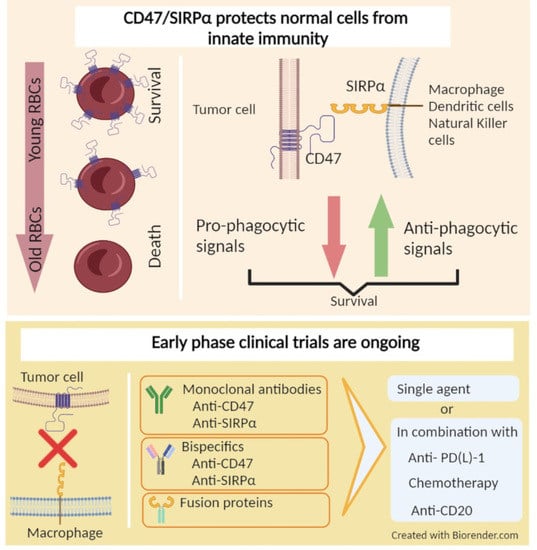
3. CD47/SIRPα Axis
4. Role of CD47/SIRPα in Cancer
5. Therapies Targeting CD47/SIRPα in Cancer
6. Anti-CD47 Antibodies and CD47-Targeting Recombinant Proteins
7. SIRPα Targeting Agents
8. Bispecific Agents Targeting CD47 and Another Molecule
9. Other Approaches Targeting CD47/SIRPα Axis
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients with Metastatic Melanoma: Analysis of 270 Patients Treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Strawderman, M.H.; Ernstoff, M.S.; Smith, T.J.; Borden, E.C.; Blum, R.H. Interferon Alfa-2b Adjuvant Therapy of High-Risk Resected Cutaneous Melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol. 1996, 14, 7–17. [Google Scholar] [CrossRef]
- Hodi, F.S.; Oble, D.A.; Drappatz, J.; Velazquez, E.F.; Ramaiya, N.; Ramakrishna, N.; Day, A.L.; Kruse, A.; Mac Rae, S.; Hoos, A.; et al. CTLA-4 Blockade with Ipilimumab Induces Significant Clinical Benefit in a Female with Melanoma Metastases to the CNS. Nat. Clin. Pract. Oncol. 2008, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients with Advanced Melanoma Receiving Nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Sarnaik, A.A.; Hamid, O.; Khushalani, N.I.; Lewis, K.D.; Medina, T.; Kluger, H.M.; Thomas, S.S.; Domingo-Musibay, E.; Pavlick, A.C.; Whitman, E.D.; et al. Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. J. Clin. Oncol. 2021, 39, 2656–2666. [Google Scholar] [CrossRef]
- Hou, A.J.; Chen, L.C.; Chen, Y.Y. Navigating CAR-T Cells through the Solid-Tumour Microenvironment. Nat. Rev. Drug Discov. 2021, 20, 531–550. [Google Scholar] [CrossRef]
- Poschke, I.; Mougiakakos, D.; Kiessling, R. Camouflage and Sabotage: Tumor Escape from the Immune System. Cancer Immunol. Immunother. 2011, 60, 1161–1171. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jamieson, C.H.M.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic Immunity in Cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel Immune Checkpoint Targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Goldrath, A.W.; Bevan, M.J. Selecting and Maintaining a Diverse T-Cell Repertoire. Nature 1999, 402, 255–262. [Google Scholar] [CrossRef]
- Fife, B.T.; Bluestone, J.A. Control of Peripheral T-Cell Tolerance and Autoimmunity via the CTLA-4 and PD-1 Pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Albukhari, A.; Alsaadi, A.; Artibani, M.; Brenton, J.D.; Curbishley, S.M.; Dong, T.; Dustin, M.L.; Hu, Z.; McGranahan, N.; et al. Promises and Challenges of Adoptive T-Cell Therapies for Solid Tumours. Br. J. Cancer 2021, 124, 1759–1776. [Google Scholar] [CrossRef]
- Nowicki, A.; Szenajch, J.; Ostrowska, G.; Wojtowicz, A.; Wojtowicz, K.; Kruszewski, A.A.; Maruszynski, M.; Aukerman, S.L.; Wiktor-Jedrzejczak, W. Impaired Tumor Growth in Colony-Stimulating Factor 1 (CSF-1)-Deficient, Macrophage-Deficient Op/Op Mouse: Evidence for a Role of CSF-1-Dependent Macrophages in Formation of Tumor Stroma. Int. J. Cancer 1996, 65, 112–119. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Bingle, L.; Brown, N.J.; Lewis, C.E. The Role of Tumour-Associated Macrophages in Tumour Progression: Implications for New Anticancer Therapies. J. Pathol. 2002, 196, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Nguyen, A.V.; Russell, R.G.; Pollard, J.W. Colony-Stimulating Factor 1 Promotes Progression of Mammary Tumors to Malignancy. J. Exp. Med. 2001, 193, 727–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.J.; Frazier, W.A. Integrin-Associated Protein (CD47) and Its Ligands. Trends Cell Biol. 2001, 11, 130–135. [Google Scholar] [CrossRef]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.; Hilarius-Stokman, P.; de Korte, D.; van den Berg, T.K.; van Bruggen, R. CD47 Functions as a Molecular Switch for Erythrocyte Phagocytosis. Blood 2012, 119, 5512–5521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, A.N.; Brown, M.H. The SIRP Family of Receptors and Immune Regulation. Nat. Rev. Immunol. 2006, 6, 457–464. [Google Scholar] [CrossRef]
- Kurihara, H.; Harita, Y.; Ichimura, K.; Hattori, S.; Sakai, T. SIRP-Alpha-CD47 System Functions as an Intercellular Signal in the Renal Glomerulus. Am. J. Physiol. Renal Physiol. 2010, 299, F517–F527. [Google Scholar] [CrossRef] [PubMed]
- Matlung, H.L.; Szilagyi, K.; Barclay, N.A.; van den Berg, T.K. The CD47-SIRPα Signaling Axis as an Innate Immune Checkpoint in Cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef]
- Mawby, W.J.; Holmes, C.H.; Anstee, D.J.; Spring, F.A.; Tanner, M.J.A. Isolation and Characterization of CD47 Glycoprotein: A Multispanning Membrane Protein Which Is the Same as Integrin-Associated Protein (IAP) and the Ovarian Tumour Marker OA3. Biochem. J. 1994, 304, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Yu, G.-T.; Deng, W.-W.; Mao, L.; Yang, L.-L.; Ma, S.-R.; Bu, L.-L.; Kulkarni, A.B.; Zhang, W.-F.; Zhang, L.; et al. Anti-CD47 Treatment Enhances Anti-Tumor T-Cell Immunity and Improves Immunosuppressive Environment in Head and Neck Squamous Cell Carcinoma. OncoImmunology 2018, 7, e1397248. [Google Scholar] [CrossRef]
- Chao, M.P.; Tang, C.; Pachynski, R.K.; Chin, R.; Majeti, R.; Weissman, I.L. Extranodal Dissemination of Non-Hodgkin Lymphoma Requires CD47 and Is Inhibited by Anti-CD47 Antibody Therapy. Blood 2011, 118, 4890–4901. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.-P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-Signal Regulatory Protein Alpha (SIRPa) Interaction Is a Therapeutic Target for Human Solid Tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molecular Pathways: Activating T Cells after Cancer Cell Phagocytosis from Blockade of CD47 “Don’t Eat Me” Signals|Clinical Cancer Research. Available online: https://clincancerres.aacrjournals.org/content/21/16/3597 (accessed on 24 August 2021).
- Weiskopf, K.; Jahchan, N.S.; Schnorr, P.J.; Cristea, S.; Ring, A.M.; Maute, R.L.; Volkmer, A.K.; Volkmer, J.-P.; Liu, J.; Lim, J.S.; et al. CD47-Blocking Immunotherapies Stimulate Macrophage-Mediated Destruction of Small-Cell Lung Cancer. J. Clin. Investig. 2016, 126, 2610–2620. [Google Scholar] [CrossRef]
- Edris, B.; Weiskopf, K.; Volkmer, A.K.; Volkmer, J.-P.; Willingham, S.B.; Contreras-Trujillo, H.; Liu, J.; Majeti, R.; West, R.B.; Fletcher, J.A.; et al. Antibody Therapy Targeting the CD47 Protein Is Effective in a Model of Aggressive Metastatic Leiomyosarcoma. Proc. Natl. Acad. Sci. USA 2012, 109, 6656–6661. [Google Scholar] [CrossRef] [Green Version]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wang, S.; Li, J.; Li, B. CD47/SIRPα Blocking Enhances CD19/CD3-Bispecific T Cell Engager Antibody-Mediated Lysis of B Cell Malignancies. Biochem. Biophys. Res. Commun. 2019, 509, 739–745. [Google Scholar] [CrossRef]
- Yoshida, K.; Tsujimoto, H.; Matsumura, K.; Kinoshita, M.; Takahata, R.; Matsumoto, Y.; Hiraki, S.; Ono, S.; Seki, S.; Yamamoto, J.; et al. CD47 Is an Adverse Prognostic Factor and a Therapeutic Target in Gastric Cancer. Cancer Med. 2015, 4, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Chung, H.; Banan, B.; Manning, P.T.; Ott, K.C.; Lin, S.; Capoccia, B.J.; Subramanian, V.; Hiebsch, R.R.; Upadhya, G.A.; et al. Antibody Mediated Therapy Targeting CD47 Inhibits Tumor Progression of Hepatocellular Carcinoma. Cancer Lett. 2015, 360, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ring, N.G.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.-P.; George, B.M.; Lietzenmayer, M.; McKenna, K.M.; Naik, T.J.; McCarty, A.; et al. Anti-SIRPα Antibody Immunotherapy Enhances Neutrophil and Macrophage Antitumor Activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaeteewoottacharn, K.; Kariya, R.; Pothipan, P.; Fujikawa, S.; Pairojkul, C.; Waraasawapati, S.; Kuwahara, K.; Wongkham, C.; Wongkham, S.; Okada, S. Attenuation of CD47-SIRPα Signal in Cholangiocarcinoma Potentiates Tumor-Associated Macrophage-Mediated Phagocytosis and Suppresses Intrahepatic Metastasis. Transl. Oncol. 2018, 12, 217–225. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) as Major Players of the Cancer-Related Inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hutter, G.; Kahn, S.A.; Azad, T.D.; Gholamin, S.; Xu, C.Y.; Liu, J.; Achrol, A.S.; Richard, C.; Sommerkamp, P.; et al. Anti-CD47 Treatment Stimulates Phagocytosis of Glioblastoma by M1 and M2 Polarized Macrophages and Promotes M1 Polarized Macrophages In Vivo. PLoS ONE 2016, 11, e0153550. [Google Scholar] [CrossRef]
- Nath, P.R.; Pal-Nath, D.; Mandal, A.; Cam, M.C.; Schwartz, A.L.; Roberts, D.D. Natural Killer Cell Recruitment and Activation Are Regulated by CD47 Expression in the Tumor Microenvironment. Cancer Immunol. Res. 2019, 7, 1547–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuse, T.; Hu, X.; Agbor-Enoh, S.; Jang, M.K.; Alawi, M.; Saygi, C.; Gravina, A.; Tediashvili, G.; Nguyen, V.Q.; Liu, Y.; et al. The SIRPα–CD47 Immune Checkpoint in NK Cells. J. Exp. Med. 2021, 218, e20200839. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.-C.; Lee, J.-J.; Kim, S.; Lee, S.G.; Park, S.-W.; Sung, M.W.; Heo, D.S. Association of CD47 with Natural Killer Cell-Mediated Cytotoxicity of Head-and-Neck Squamous Cell Carcinoma Lines. Tumor Biol. 2008, 29, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Veillette, A.; Chen, J. SIRPα–CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Uno, S.; Kinoshita, Y.; Yoshimura, Y.; Iida, S.-I.; Wakahara, Y.; Tsuchiya, M.; Yamada-Okabe, H.; Fukushima, N. Apoptosis Inducing Bivalent Single-Chain Antibody Fragments against CD47 Showed Antitumor Potency for Multiple Myeloma. Leuk Res. 2005, 29, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Boukhari, A.; Alhosin, M.; Bronner, C.; Sagini, K.; Truchot, C.; Sick, E.; Schini-Kerth, V.B.; André, P.; Mély, Y.; Mousli, M.; et al. CD47 Activation-Induced UHRF1 over-Expression Is Associated with Silencing of Tumor Suppressor Gene P16INK4A in Glioblastoma Cells. Anticancer Res. 2015, 35, 149–157. [Google Scholar]
- Uluçkan, O.; Becker, S.N.; Deng, H.; Zou, W.; Prior, J.L.; Piwnica-Worms, D.; Frazier, W.A.; Weilbaecher, K.N. CD47 Regulates Bone Mass and Tumor Metastasis to Bone. Cancer Res. 2009, 69, 3196–3204. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Sun, L.; Yuan, X.; Qiu, H. Thrombospondin-1 Is a Multifaceted Player in Tumor Progression. Oncotarget 2017, 8, 84546–84558. [Google Scholar] [CrossRef] [Green Version]
- Byrne, G.J.; Hayden, K.E.; McDowell, G.; Lang, H.; Kirwan, C.C.; Tetlow, L.; Kumar, S.; Bundred, N.J. Angiogenic Characteristics of Circulating and Tumoural Thrombospondin-1 in Breast Cancer. Int. J. Oncol. 2007, 31, 1127–1132. [Google Scholar] [CrossRef] [Green Version]
- Borsotti, P.; Ghilardi, C.; Ostano, P.; Silini, A.; Dossi, R.; Pinessi, D.; Foglieni, C.; Scatolini, M.; Lacal, P.M.; Ferrari, R.; et al. Thrombospondin-1 Is Part of a Slug-Independent Motility and Metastatic Program in Cutaneous Melanoma, in Association with VEGFR-1 and FGF-2. Pigment. Cell Melanoma Res. 2015, 28, 73–81. [Google Scholar] [CrossRef]
- Kamijo, H.; Miyagaki, T.; Takahashi-Shishido, N.; Nakajima, R.; Oka, T.; Suga, H.; Sugaya, M.; Sato, S. Thrombospondin-1 Promotes Tumor Progression in Cutaneous T-Cell Lymphoma via CD47. Leukemia 2020, 34, 845–856. [Google Scholar] [CrossRef]
- Liu, X.; Pu, Y.; Cron, K.; Deng, L.; Kline, J.; Frazier, W.A.; Xu, H.; Peng, H.; Fu, Y.-X.; Xu, M.M. CD47 Blockade Triggers T Cell-Mediated Destruction of Immunogenic Tumors. Nat. Med. 2015, 21, 1209–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Harrabi, O.; Fong, A.P.; Ruffner, K.L.; Forgie, A.J.; Sim, J.; Randolph, S.S.; Wan, H.; Pons, J.; Kuo, T.C. ALX148 Enhances the Depth and Durability of Response to Multiple AML Therapies. Blood 2020, 136, 15–16. [Google Scholar] [CrossRef]
- Tseng, D.; Volkmer, J.-P.; Willingham, S.B.; Contreras-Trujillo, H.; Fathman, J.W.; Fernhoff, N.B.; Seita, J.; Inlay, M.A.; Weiskopf, K.; Miyanishi, M.; et al. Anti-CD47 Antibody-Mediated Phagocytosis of Cancer by Macrophages Primes an Effective Antitumor T-Cell Response. Proc. Natl. Acad. Sci. USA 2013, 110, 11103–11108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Shao, R.; Huang, H.; Wang, X.; Rong, Z.; Lin, Y. Engineering Macrophages to Phagocytose Cancer Cells by Blocking the CD47/SIRPɑ Axis. Cancer Med. 2019, 8, 4245–4253. [Google Scholar] [CrossRef] [Green Version]
- Gauttier, V.; Pengam, S.; Durand, J.; Biteau, K.; Mary, C.; Morello, A.; Néel, M.; Porto, G.; Teppaz, G.; Thepenier, V.; et al. Selective SIRPα Blockade Reverses Tumor T Cell Exclusion and Overcomes Cancer Immunotherapy Resistance. J. Clin. Investig. 2020, 130, 6109–6123. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pantoja, D.R.; Terabe, M.; Ghosh, A.; Ridnour, L.A.; DeGraff, W.G.; Wink, D.A.; Berzofsky, J.A.; Roberts, D.D. CD47 in the Tumor Microenvironment Limits Cooperation between Antitumor T-Cell Immunity and Radiotherapy. Cancer Res. 2014, 74, 6771–6783. [Google Scholar] [CrossRef] [Green Version]
- Von Roemeling, C.A.; Wang, Y.; Qie, Y.; Yuan, H.; Zhao, H.; Liu, X.; Yang, Z.; Yang, M.; Deng, W.; Bruno, K.A.; et al. Therapeutic Modulation of Phagocytosis in Glioblastoma Can Activate Both Innate and Adaptive Antitumour Immunity. Nat. Commun. 2020, 11, 1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Torres, A.-C.; Quiney, C.; Attout, T.; Boullet, H.; Herbi, L.; Vela, L.; Barbier, S.; Chateau, D.; Chapiro, E.; Nguyen-Khac, F.; et al. CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans. PLoS Med. 2015, 12, e1001796. [Google Scholar] [CrossRef]
- Tao, H.; Qian, P.; Wang, F.; Yu, H.; Guo, Y. Targeting CD47 Enhances the Efficacy of Anti-PD-1 and CTLA-4 in an Esophageal Squamous Cell Cancer Preclinical Model. Oncol. Res. 2017, 25, 1579–1587. [Google Scholar] [CrossRef]
- Nagahara, M.; Mimori, K.; Kataoka, A.; Ishii, H.; Tanaka, F.; Nakagawa, T.; Sato, T.; Ono, S.; Sugihara, K.; Mori, M. Correlated Expression of CD47 and SIRPA in Bone Marrow and in Peripheral Blood Predicts Recurrence in Breast Cancer Patients. Clin. Cancer Res. 2010, 16, 4625–4635. [Google Scholar] [CrossRef] [Green Version]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.; Zlobec, I.; Schürch, C.; Perren, A.; Ochsenbein, A.F.; Banz, Y. CD47 Protein Expression in Acute Myeloid Leukemia: A Tissue Microarray-Based Analysis. Leuk Res. 2015, 39, 749–756. [Google Scholar] [CrossRef]
- Fu, W.; Li, J.; Zhang, W.; Li, P. High Expression of CD47 Predicts Adverse Prognosis in Chinese Patients and Suppresses Immune Response in Melanoma. Biomed. Pharmacother. 2017, 93, 1190–1196. [Google Scholar] [CrossRef]
- Overexpression of CD47 Predicts Poor Prognosis and Promotes Cancer Cell Invasion in High-Grade Serous Ovarian Carcinoma. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5489890/ (accessed on 24 August 2021).
- Upton, R.; Banuelos, A.; Feng, D.; Biswas, T.; Kao, K.; McKenna, K.; Willingham, S.; Ho, P.Y.; Rosental, B.; Tal, M.C.; et al. Combining CD47 Blockade with Trastuzumab Eliminates HER2-Positive Breast Cancer Cells and Overcomes Trastuzumab Tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026849118. [Google Scholar] [CrossRef]
- Briere, D.; Sudhakar, N.; Woods, D.M.; Hallin, J.; Engstrom, L.D.; Aranda, R.; Chiang, H.; Sodré, A.L.; Olson, P.; Weber, J.S.; et al. The Class I/IV HDAC Inhibitor Mocetinostat Increases Tumor Antigen Presentation, Decreases Immune Suppressive Cell Types and Augments Checkpoint Inhibitor Therapy. Cancer Immunol. Immunother. 2018, 67, 381–392. [Google Scholar] [CrossRef]
- Orillion, A.; Hashimoto, A.; Damayanti, N.; Shen, L.; Adelaiye-Ogala, R.; Arisa, S.; Chintala, S.; Ordentlich, P.; Kao, C.; Elzey, B.; et al. Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumor Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 5187–5201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catani, L.; Sollazzo, D.; Ricci, F.; Polverelli, N.; Palandri, F.; Baccarani, M.; Vianelli, N.; Lemoli, R.M. The CD47 Pathway Is Deregulated in Human Immune Thrombocytopenia. Exp. Hematol. 2011, 39, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, S.; van Rooijen, N.; Saxena, R.K. Reduced Expression of CD47 during Murine Red Blood Cell (RBC) Senescence and Its Role in RBC Clearance from the Circulation. Transfusion 2007, 47, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, C.H.; Chao, M.P.; Gibbs, C.; McCamish, M.A.; Liu, J.; Chen, J.Y.; Majeti, R.; Weissman, I.L. The Macrophage ‘Do Not Eat Me’ Signal, CD47, Is a Clinically Validated Cancer Immunotherapy Target. Ann. Oncol. 2019, 30, 486–489. [Google Scholar] [CrossRef]
- Chao, M.P.; Majeti, R.; Weissman, I.L. Programmed Cell Removal: A New Obstacle in the Road to Developing Cancer. Nat. Rev. Cancer 2012, 12, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholamin, S.; Mitra, S.S.; Feroze, A.H.; Liu, J.; Kahn, S.A.; Zhang, M.; Esparza, R.; Richard, C.; Ramaswamy, V.; Remke, M.; et al. Disrupting the CD47-SIRPα Anti-Phagocytic Axis by a Humanized Anti-CD47 Antibody Is an Efficacious Treatment for Malignant Pediatric Brain Tumors. Sci. Transl. Med. 2017, 9, eaaf2968. [Google Scholar] [CrossRef] [Green Version]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’Rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients with Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef]
- Brierley, C.K.; Staves, J.; Roberts, C.; Johnson, H.; Vyas, P.; Goodnough, L.T.; Murphy, M.F. The Effects of Monoclonal Anti-CD47 on RBCs, Compatibility Testing, and Transfusion Requirements in Refractory Acute Myeloid Leukemia. Transfusion 2019, 59, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, N.; Orloff, M.; Fu, S.; Liu, Y.; Wang, Y.; Zhou, H.; Lin, K.; Liu, F.; Yan, S.; Patnaik, A. 295 First-in-Human Phase I Trial of IBI188, an Anti-CD47 Targeting Monoclonal Antibody, in Patients with Advanced Solid Tumors and Lymphomas. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Gan, H.K.; Coward, J.; Mislang, A.R.A.; Cosman, R.; Nagrial, A.; Jin, X.; Li, B.; Wang, Z.M.; Kwek, K.Y.; Xia, D.; et al. Safety of AK117, an Anti-CD47 Monoclonal Antibody, in Patients with Advanced or Metastatic Solid Tumors in a Phase I Study. J. Clin. Oncol. 2021, 39, 2630. [Google Scholar] [CrossRef]
- Ansell, S.M.; Flinn, I.W.; Maris, M.B.; O’Connor, O.A.; Lesokhin, A.; Advani, A.S.; Minden, M.D.; Percival, M.B.M.; Johnson, L.D.; Catalano, T.; et al. TTI-621 (SIRPαFc), an Immune Checkpoint Inhibitor Blocking the CD47 “Do Not Eat” Signal, Induces Objective Responses in Patients with Advanced, Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2017, 130, 4116. [Google Scholar] [CrossRef]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.-E.M.; Advani, A.S.; et al. Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef]
- Querfeld, C.; Thompson, J.A.; Taylor, M.; Pillai, R.; Johnson, L.D.S.; Catalano, T.; Petrova, P.S.; Uger, B.A.; Irwin, M.; Thompson, T.; et al. Intralesional Injection of the CD47-Blocking Immune Checkpoint Inhibitor TTI-621 (SIRPaFc) Induces Antitumor Activity in Patients with Relapsed/Refractory Mycosis Fungoides and Sézary Syndrome: Interim Results of a Multicenter Phase 1 Trial. Eur. J. Cancer 2018, 101, S34. [Google Scholar] [CrossRef]
- Patel, K. Investigational CD47-Blocker TTI-622 Shows Single-Agent Activity in Patients with Advanced Relapsed or Refractory Lymphoma: Update from the Ongoing First-in-Human Dose Escalation Study. Blood 2020, 136, 46–47. [Google Scholar] [CrossRef]
- Weiskopf, K.; Ring, A.M.; Ho, C.C.M.; Volkmer, J.-P.; Levin, A.M.; Volkmer, A.K.; Ozkan, E.; Fernhoff, N.B.; van de Rijn, M.; Weissman, I.L.; et al. Engineered SIRPα Variants as Immunotherapeutic Adjuvants to Anticancer Antibodies. Science 2013, 341, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Lakhani, N.J.; LoRusso, P.; Hafez, N.; Krishnamurthy, A.; O’Rourke, T.J.; Kamdar, M.K.; Fanning, P.; Zhao, Y.; Jin, F.; Wan, H.; et al. A Phase 1 Study of ALX148, a CD47 Blocker, Alone and in Combination with Established Anticancer Antibodies in Patients with Advanced Malignancy and Non-Hodgkin Lymphoma. J. Clin. Oncol. 2018, 36, 3068. [Google Scholar] [CrossRef]
- Kauder, S.E.; Kuo, T.C.; Harrabi, O.; Chen, A.; Sangalang, E.; Doyle, L.; Rocha, S.S.; Bollini, S.; Han, B.; Sim, J.; et al. ALX148 Blocks CD47 and Enhances Innate and Adaptive Antitumor Immunity with a Favorable Safety Profile. PLoS ONE 2018, 13, e0201832. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.M.; Laknahi, N.; Gainor, J.; Kamdar, M.; Fanning, P.; Squifflet, P.; Jin, F.; Forgie, A.J.; Wan, H.; Pons, J.; et al. ALX148, a CD47 Blocker, in Combination with Rituximab in Patients with Non-Hodgkin Lymphoma. Blood 2020, 136, 13–14. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Gainor, J.F.; Lakhani, N.J.; Lee, K.W.; Chung, H.C.; Lee, J.; LoRusso, P.; Bang, Y.-J.; Hodi, F.S.; Santana-Davila, R.; et al. A Phase I Study of ALX148, a CD47 Blocker, in Combination with Standard Anticancer Antibodies and Chemotherapy Regimens in Patients with Advanced Malignancy. J. Clin. Oncol. 2020, 38, 3056. [Google Scholar] [CrossRef]
- Adams, K.F.; Leitzmann, M.F.; Albanes, D.; Kipnis, V.; Moore, S.C.; Schatzkin, A.; Chow, W.-H. Body Size and Renal Cell Cancer Incidence in a Large US Cohort Study. Am. J. Epidemiol. 2008, 168, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Li, F.; Yang, Y.; Chen, J.; Hu, X. SIRP/CD47 Signaling in Neurological Disorders. Brain Res. 2015, 1623, 74–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanidakis, M.; Newton, G.; Lee, W.Y.; Parkos, C.A.; Luscinskas, F.W. Endothelial CD47 Interaction with SIRPgamma Is Required for Human T-Cell Transendothelial Migration under Shear Flow Conditions in Vitro. Blood 2008, 112, 1280–1289. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, A.; Ohnishi, H.; Okazawa, H.; Nakazawa, S.; Ikeda, H.; Motegi, S.; Aoki, N.; Kimura, S.; Mikuni, M.; Matozaki, T. Positive Regulation of Phagocytosis by SIRPbeta and Its Signaling Mechanism in Macrophages. J. Biol. Chem. 2004, 279, 29450–29460. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Soto, I.; Tong, Q.; Chin, A.; Bühring, H.-J.; Wu, T.; Zen, K.; Parkos, C.A. SIRPbeta1 Is Expressed as a Disulfide-Linked Homodimer in Leukocytes and Positively Regulates Neutrophil Transepithelial Migration. J. Biol. Chem. 2005, 280, 36132–36140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccio, L.; Vermi, W.; Boles, K.S.; Fuchs, A.; Strader, C.A.; Facchetti, F.; Cella, M.; Colonna, M. Adhesion of Human T Cells to Antigen-Presenting Cells through SIRPβ2-CD47 Interaction Costimulates T-Cell Proliferation. Blood 2005, 105, 2421–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forty Seven to Gilead: “Eat Me”. Nat. Biotechnol. 2020, 38, 389. [CrossRef] [PubMed] [Green Version]
- Delord, J.-P.; Kotecki, N.; Marabelle, A.; Vinceneux, A.; Korakis, I.; Jungels, C.; Champiat, S.; Huhn, R.D.; Poirier, N.; Costantini, D.; et al. A Phase 1 Study Evaluating BI 765063, a First in Class Selective Myeloid Sirpa Inhibitor, As Stand-Alone and in Combination with BI 754091, a Programmed Death-1 (PD-1) Inhibitor, in Patients with Advanced Solid Tumours. Blood 2019, 134, 1040. [Google Scholar] [CrossRef]
- Champiat, S.; Cassier, P.A.; Kotecki, N.; Korakis, I.; Vinceneux, A.; Jungels, C.; Blatchford, J.; Elgadi, M.M.; Clarke, N.; Fromond, C.; et al. Safety, Pharmacokinetics, Efficacy, and Preliminary Biomarker Data of First-in-Class BI 765063, a Selective SIRPα Inhibitor: Results of Monotherapy Dose Escalation in Phase 1 Study in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2021, 39, 2623. [Google Scholar] [CrossRef]
- Voets, E.; Paradé, M.; Lutje Hulsik, D.; Spijkers, S.; Janssen, W.; Rens, J.; Reinieren-Beeren, I.; van den Tillaart, G.; van Duijnhoven, S.; Driessen, L.; et al. Functional Characterization of the Selective Pan-Allele Anti-SIRPα Antibody ADU-1805 That Blocks the SIRPα-CD47 Innate Immune Checkpoint. J. Immunother. Cancer 2019, 7, 340. [Google Scholar] [CrossRef] [PubMed]
- Ho CC, M.; Guo, N.; Sockolosky, J.T.; Ring, A.M.; Weiskopf, K.; Özkan, E.; Mori, Y.; Weissman, I.L.; Garcia, K.C. “Velcro” Engineering of High Affinity CD47 Ectodomain as Signal Regulatory Protein α (SIRPα) Antagonists That Enhance Antibody-Dependent Cellular Phagocytosis. J. Biol. Chem. 2015, 290, 12650–12663. [Google Scholar]
- Andrejeva, G.; Capoccia, B.J.; Hiebsch, R.R.; Donio, M.J.; Darwech, I.M.; Puro, R.J.; Pereira, D.S. Novel SIRPα Antibodies That Induce Single-Agent Phagocytosis of Tumor Cells While Preserving T Cells. J. Immunol. 2021, 206, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Sockolosky, J.T.; Sangalang, E.; Izquierdo, S.; Pedersen, D.; Harriman, W.; Wibowo, A.S.; Carter, J.; Madan, A.; Doyle, L.; et al. Discovery of High Affinity, Pan-Allelic, and Pan-Mammalian Reactive Antibodies against the Myeloid Checkpoint Receptor SIRPα. MAbs 2019, 11, 1036–1052. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, L.; Ren, Z.; Yang, K.; Xu, H.; Luan, Y.; Fu, K.; Guo, J.; Peng, H.; Zhu, M.; et al. Dual Targeting of Innate and Adaptive Checkpoints on Tumor Cells Limits Immune Evasion. Cell Rep. 2018, 24, 2101–2111. [Google Scholar] [CrossRef] [Green Version]
- Piccione, E.C.; Juarez, S.; Liu, J.; Tseng, S.; Ryan, C.E.; Narayanan, C.; Wang, L.; Weiskopf, K.; Majeti, R. A Bispecific Antibody Targeting CD47 and CD20 Selectively Binds and Eliminates Dual Antigen Expressing Lymphoma Cells. MAbs 2015, 7, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Roohullah, A.; Ganju, V.; Zhang, F.; Zhang, L.; Yu, T.; Wilkinson, K.; Cooper, A.; de Souza, P. First-in-Human Phase 1 Dose Escalation Study of HX009, a Novel Recombinant Humanized Anti-PD-1 and CD47 Bispecific Antibody, in Patients with Advanced Malignancies. J. Clin. Oncol. 2021, 39, 2517. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, M.; Lin, P.; Liu, J.; Liu, C.; Strohl, W.R.; Wang, S.; Ho, M. Frontiers and Opportunities: Highlights of the 2nd Annual Conference of the Chinese Antibody Society. Antib. Ther. 2018, 1, 27–36. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, D.; Huang, C.; Chen, B.; Li, M.; Zhou, S.; Wang, L.; Wu, M.; Wang, X.; Bian, Y.; et al. Dose Escalation PET Imaging for Safety and Effective Therapy Dose Optimization of a Bispecific Antibody. MAbs 2020, 12, 1748322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ni, H.; Zhou, S.; He, K.; Gao, Y.; Wu, W.; Wu, M.; Wu, Z.; Qiu, X.; Zhou, Y.; et al. Tumor-Selective Blockade of CD47 Signaling with a CD47/PD-L1 Bispecific Antibody for Enhanced Anti-Tumor Activity and Limited Toxicity. Cancer Immunol. Immunother. 2021, 70, 365–376. [Google Scholar] [CrossRef] [PubMed]
- 33rd Annual Meeting & Pre-Conference Programs of the Society for Immunotherapy of Cancer (SITC 2018). J. Immunother. Cancer 2018, 6, 115. [CrossRef] [Green Version]
- Djureinovic, D.; Wang, M.; Kluger, H.M. Agonistic CD40 Antibodies in Cancer Treatment. Cancers 2021, 13, 1302. [Google Scholar] [CrossRef]
- De Silva, S.; Fromm, G.; Shuptrine, C.W.; Johannes, K.; Patel, A.; Yoo, K.J.; Huang, K.; Schreiber, T.H. CD40 Enhances Type I Interferon Responses Downstream of CD47 Blockade, Bridging Innate and Adaptive Immunity. Cancer Immunol. Res. 2020, 8, 230–245. [Google Scholar] [CrossRef] [Green Version]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular Mechanism and Function of CD40/CD40L Engagement in the Immune System. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Li, S.; Chen, D.; Liang, G.; Zhang, L.; Zhang, W.; Tu, X.; Peng, L.; Weng, J.; Zhao, G.; et al. Abstract 545: Preclinical Development of a Bispecific Antibody-Trap Selectively Targeting CD47 and CD20 for the Treatment of B Cell Lineage Cancer. Cancer Res. 2019, 79, 545. [Google Scholar] [CrossRef]
- Buatois, V.; Johnson, Z.; Salgado-Pires, S.; Papaioannou, A.; Hatterer, E.; Chauchet, X.; Richard, F.; Barba, L.; Daubeuf, B.; Cons, L.; et al. Preclinical Development of a Bispecific Antibody That Safely and Effectively Targets CD19 and CD47 for the Treatment of B-Cell Lymphoma and Leukemia. Mol. Cancer Ther. 2018, 17, 1739–1751. [Google Scholar] [CrossRef] [Green Version]
- Golubovskaya, V.; Berahovich, R.; Zhou, H.; Xu, S.; Harto, H.; Li, L.; Chao, C.-C.; Mao, M.M.; Wu, L. CD47-CAR-T Cells Effectively Kill Target Cancer Cells and Block Pancreatic Tumor Growth. Cancers 2017, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- La, H.T.; Tran, D.B.T.; Tran, H.M.; Nguyen, L.T. Third-Generation Anti-CD47-Specific CAR-T Cells Effectively Kill Cancer Cells and Reduce the Genes Expression in Lung Cancer Cell Metastasis. J. Immunol. Res. 2021, 2021, e5575260. [Google Scholar] [CrossRef]
- Shu, R.; Evtimov, V.J.; Hammett, M.V.; Nguyen, N.-Y.N.; Zhuang, J.; Hudson, P.J.; Howard, M.C.; Pupovac, A.; Trounson, A.O.; Boyd, R.L. Engineered CAR-T Cells Targeting TAG-72 and CD47 in Ovarian Cancer. Mol. Ther. Oncolytics 2021, 20, 325–341. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, M.; Gai, J.; Li, G.; Chang, Q.; Qiao, P.; Cao, L.; Chen, W.; Zhang, S.; Wan, Y. Preclinical Development of a Novel CD47 Nanobody with Less Toxicity and Enhanced Anti-Cancer Therapeutic Potential. J. Nanobiotechnol. 2020, 18, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In Situ Sprayed Bioresponsive Immunotherapeutic Gel for Post-Surgical Cancer Treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Castro, S.; Coker, C.; Hinchliffe, T.E.; Arpaia, N.; Danino, T. Programmable Bacteria Induce Durable Tumor Regression and Systemic Antitumor Immunity. Nat. Med. 2019, 25, 1057–1063. [Google Scholar] [CrossRef] [PubMed]

| Agent | Therapeutic Target | Design | Phase | Disease Site | Accrual Goal | Identifier |
|---|---|---|---|---|---|---|
| Monoclonal Antibodies | ||||||
| IBI188 (Letaplimab) | CD47 | IBI188 +/− rituximab | I | Metastatic solid tumors or lymphoma | 92 | NCT03717103 |
| IBI188 +/− azacitidine | I | Myelodysplastic syndrome | 12 | NCT04485065 | ||
| Hu5F9-G4 (Magrolimab) | CD47 | Hu5F9-G4 (Magrolimab) + Pembrolizumab | II | Hodgkin’s lymphoma | 24 | NCT04788043 |
| Hu5F9-G4 (Magrolimab) | I | Hematologic malignancies | 20 | NCT02678338 | ||
| Hu5F9-G4 (Magrolimab) + acalabrutinib + rituximab or other combinations without Hu5F9-G4 (Magrolimab) | I | Non-Hodgkin’s Lymphoma | 30 | NCT03527147 | ||
| Hu5F9-G4 (Magrolimab) + Obinutuzumab + venetoclax | I | Non-Hodgkin’s Lymphoma | 76 | NCT04599634 | ||
| ZL-1201 | CD47 | ZL-1201 | I | Metastatic solid tumors or refractory lymphomas | 66 | NCT04257617 |
| STI-6643 | CD47 | STI-6643 | I | Metastatic solid tumors | 24 | NCT04900519 |
| CC-9002 | CD47 | CC-90002 +/−rituximab | Part A: Metastatic solid tumors, multiple Myeloma or non-Hodgkin’s lymphoma Part B, relapsed and/or refractory CD20-positive NHL | 60 | NCT02367196 | |
| AK117 | CD47 | AK117 | I | Metastatic solid tumors or lymphoma | 162 | NCT04728334 |
| AK117 + azacitidine | I/II | Myelodysplastic syndrome | 190 | NCT04900350 | ||
| AO-176 | CD47 | AO-176 +/− paclitaxel | I/II | Metastatic solid tumors | 132 | NCT03834948 |
| AO-176 +/− dexamethasone or dexhamethasone + bortezomide | I | Multiple myeloma | 102 | NCT04445701 | ||
| IMC-002 | CD47 | IMC-002 | I | Metastatic solid tumors or lymphoma | 24 | NCT04306224 |
| TQB2928 | CD47 | TQB2928 | I | Metastatic solid tumors or hematologic malignancies | 20 | NCT04854681 |
| FSI-189 | SIRPα | FSI-189 +/− rituximab | I | Non-Hodgkin’s lymphoma (B-cell) | 63 | NCT04502706 |
| BI 765063 | SIRPα | BI 765063 +/− PD-1 inhibitor | I | Metastatic solid tumors with SIRPα polymorphism | 116 | NCT03990233 |
| Bispecific antibodies | ||||||
| HX009 | CD47 and PD-1 | HX009 | II | Metastatic solid tumors | 210 | NCT04886271 |
| PF-07257876 | CD47 and PD-L1 | PF-07257876 | I | Non small-cell lung cancer, head and neck squamous cell carcinoma, ovarian cancer | 90 | NCT04881045 |
| CPO107 (JMP601) | CD47 and CD20 | CPO107 (JMP601) | I | Non-Hodgkin’s lymphoma (CD-20 positive) | 75 | NCT04853329 |
| IBI322 | CD47 and PD-L1 | IBI322 | I | Hematologic malignancies | 182 | NCT04795128 |
| IBI322 | Ia | Metastatic solid tumors | 45 | NCT04338659 | ||
| IBI322 | Ia/Ib | Metastatic solid tumors | 218 | NCT04328831 | ||
| SL-172154 | SIRPα and CD40L | SL-172154 (intravenous) | I | Ovarian cancer | 40 | NCT04406623 |
| SL-172154 (intratumoral) | I | Head and neck or cutaneous squamous cell carcinoma | 18 | NCT04502888 | ||
| TG-1801 | CD47 and CD19 | TG-1801 +/− ubitixumab | Ib | Hematologic malignancies | 60 | NCT04806035 |
| IMM0306 | CD47 and CD20 | IMM0306 | I | Refractory or Relapsed CD20-positive B cell Non-Hodgkin’s Lymphoma | 131 | NCT04746131 |
| Fusion proteins | ||||||
| TTI-622 | CD47 via SIRPαFc (IgG4) structure | TTI-622 + rituximab, PD-1 inhibitor, Proteasome inhibitor regimen or rituximab | Ia/Ib | Lymphoma or myeloma | 156 | NCT03530683 |
| ALX148 | CD47 via SIRPαFc (IgG1) structure | ALX148 + azacitidine | I/II | Myelodysplastic syndrome | 173 | NCT04417517 |
| ALX148 + venetoclax or azacitidine | I/II | Acute myleoid leukemia | 97 | NCT04755244 | ||
| ALX148 | II | Head and neck squamous cell carcinoma | 112 | NCT04675333 | ||
| ALX148 + pembrolizumab | II | Head and neck squamous cell carcinoma | 111 | NCT04675294 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dizman, N.; Buchbinder, E.I. Cancer Therapy Targeting CD47/SIRPα. Cancers 2021, 13, 6229. https://doi.org/10.3390/cancers13246229
Dizman N, Buchbinder EI. Cancer Therapy Targeting CD47/SIRPα. Cancers. 2021; 13(24):6229. https://doi.org/10.3390/cancers13246229
Chicago/Turabian StyleDizman, Nazli, and Elizabeth I. Buchbinder. 2021. "Cancer Therapy Targeting CD47/SIRPα" Cancers 13, no. 24: 6229. https://doi.org/10.3390/cancers13246229