Natural Killer Cells in Post-Transplant Lymphoproliferative Disorders
Abstract
:Simple Summary
Abstract
1. Introduction
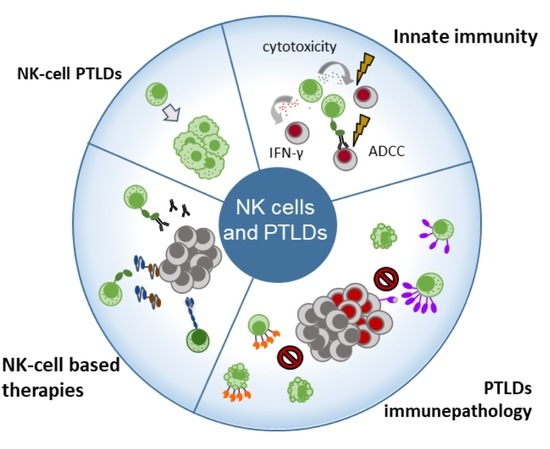
2. The Role of NK Cells in the Immunopathology of PTLDs
2.1. NK Cells and EBV-Positive PTLDs
2.2. NK Cells and EBV-Negative PTLDs
3. Putative Strategies Exploiting NK Cell Therapy to Treat PTLDs
3.1. NK Cell-Mediated ADCC
3.2. NK Cell Engagers
3.3. NK Cell Enhancement with Cytokines
3.4. Adoptive NK Cell Transfer
3.5. Chimeric Antigen-Receptor NK Cells (CAR-NK)
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Lanier, L.L.; Le, A.M.; Phillips, J.H.; Warner, N.L.; Babcock, G.F. Subpopulations of Human Natural Killer Cells Defined by Expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) Antigens. J. Immunol. 1983, 131, 1789–1796. [Google Scholar]
- Trinchieri, G. Biology of Natural Killer Cells. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 1989; Volume 47, pp. 187–376. ISBN 978-0-12-022447-0. [Google Scholar]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK Cells: Surface Receptors, Inhibitory Checkpoints, and Translational Applications. Cell Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Crome, S.Q.; Lang, P.A.; Lang, K.S.; Ohashi, P.S. Natural Killer Cells Regulate Diverse T Cell Responses. Trends Immunol. 2013, 34, 342–349. [Google Scholar] [CrossRef]
- Meza Guzman, L.G.; Keating, N.; Nicholson, S.E. Natural Killer Cells: Tumor Surveillance and Signaling. Cancers 2020, 12, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, Q.; Rückert, T.; Romagnani, C. Natural Killer Cell Specificity for Viral Infections. Nat. Immunol. 2018, 19, 800–808. [Google Scholar] [CrossRef]
- Pappworth, I.Y.; Wang, E.C.; Rowe, M. The Switch from Latent to Productive Infection in Epstein-Barr Virus-Infected B Cells Is Associated with Sensitization to NK Cell Killing. J. Virol. 2007, 81, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Azzi, T.; Lünemann, A.; Murer, A.; Ueda, S.; Béziat, V.; Malmberg, K.-J.; Staubli, G.; Gysin, C.; Berger, C.; Münz, C.; et al. Role for Early-Differentiated Natural Killer Cells in Infectious Mononucleosis. Blood 2014, 124, 2533–2543. [Google Scholar] [CrossRef] [Green Version]
- Eidenschenk, C.; Dunne, J.; Jouanguy, E.; Fourlinnie, C.; Gineau, L.; Bacq, D.; McMahon, C.; Smith, O.; Casanova, J.-L.; Abel, L.; et al. A Novel Primary Immunodeficiency with Specific Natural-Killer Cell Deficiency Maps to the Centromeric Region of Chromosome 8. Am. J. Hum. Genet. 2006, 78, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Gineau, L.; Cognet, C.; Kara, N.; Lach, F.P.; Dunne, J.; Veturi, U.; Picard, C.; Trouillet, C.; Eidenschenk, C.; Aoufouchi, S.; et al. Partial MCM4 Deficiency in Patients with Growth Retardation, Adrenal Insufficiency, and Natural Killer Cell Deficiency. J. Clin. Investig. 2012, 122, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mace, E.M.; Orange, J.S. Emerging Insights into Human Health and NK Cell Biology from the Study of NK Cell Deficiencies. Immunol. Rev. 2019, 287, 202–225. [Google Scholar] [CrossRef]
- Baiocchi, R.A.; Ward, J.S.; Carrodeguas, L.; Eisenbeis, C.F.; Peng, R.; Roychowdhury, S.; Vourganti, S.; Sekula, T.; O’Brien, M.; Moeschberger, M.; et al. GM-CSF and IL-2 Induce Specific Cellular Immunity and Provide Protection against Epstein-Barr Virus Lymphoproliferative Disorder. J. Clin. Investig. 2001, 108, 887–894. [Google Scholar] [CrossRef]
- Chijioke, O.; Müller, A.; Feederle, R.; Barros, M.H.M.; Krieg, C.; Emmel, V.; Marcenaro, E.; Leung, C.S.; Antsiferova, O.; Landtwing, V.; et al. Human Natural Killer Cells Prevent Infectious Mononucleosis Features by Targeting Lytic Epstein-Barr Virus Infection. Cell Rep. 2013, 5, 1489–1498. [Google Scholar] [CrossRef] [Green Version]
- Hatton, O.; Strauss-Albee, D.M.; Zhao, N.Q.; Haggadone, M.D.; Pelpola, J.S.; Krams, S.M.; Martinez, O.M.; Blish, C.A. NKG2A-Expressing Natural Killer Cells Dominate the Response to Autologous Lymphoblastoid Cells Infected with Epstein-Barr Virus. Front. Immunol. 2016, 7, 607. [Google Scholar] [CrossRef] [Green Version]
- Djaoud, Z.; Guethlein, L.A.; Horowitz, A.; Azzi, T.; Nemat-Gorgani, N.; Olive, D.; Nadal, D.; Norman, P.J.; Münz, C.; Parham, P. Two Alternate Strategies for Innate Immunity to Epstein-Barr Virus: One Using NK Cells and the Other NK Cells and Γδ T Cells. J. Exp. Med. 2017, 214, 1827–1841. [Google Scholar] [CrossRef] [Green Version]
- Mbiribindi, B.; Pena, J.K.; Arvedson, M.P.; Moreno Romero, C.; McCarthy, S.R.; Hatton, O.L.; Esquivel, C.O.; Martinez, O.M.; Krams, S.M. Epstein-Barr Virus Peptides Derived from Latent Cycle Proteins Alter NKG2A + NK Cell Effector Function. Sci. Rep. 2020, 10, 19973. [Google Scholar] [CrossRef]
- Münz, C. Tumor Microenvironment Conditioning by Abortive Lytic Replication of Oncogenic γ-Herpesviruses. Adv. Exp. Med. Biol. 2020, 1225, 127–135. [Google Scholar] [CrossRef]
- Dierickx, D.; Habermann, T.M. Post-Transplantation Lymphoproliferative Disorders in Adults. N. Engl. J. Med. 2018, 378, 549–562. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Webster, A.C.; Martinez, O.M.; Preiksaitis, J.K.; Leblond, V.; Choquet, S. Post-Transplant Lymphoproliferative Disorders. Nat. Rev. Dis. Primers 2016, 2, 15088. [Google Scholar] [CrossRef] [PubMed]
- Leblond, V.; Davi, F.; Charlotte, F.; Dorent, R.; Bitker, M.O.; Sutton, L.; Gandjbakhch, I.; Binet, J.L.; Raphael, M. Posttransplant Lymphoproliferative Disorders Not Associated with Epstein-Barr Virus: A Distinct Entity? J. Clin. Oncol. 1998, 16, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Luskin, M.R.; Heil, D.S.; Tan, K.S.; Choi, S.; Stadtmauer, E.A.; Schuster, S.J.; Porter, D.L.; Vonderheide, R.H.; Bagg, A.; Heitjan, D.F.; et al. The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. Am. J. Transplant. 2015, 15, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Martinez, O.M.; Krams, S.M. The Immune Response to Epstein Barr Virus and Implications for Posttransplant Lymphoproliferative Disorder. Transplantation 2017, 101, 2009–2016. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, S.H. T-Cell and NK-Cell Posttransplantation Lymphoproliferative Disorders. Am. J. Clin. Pathol. 2007, 127, 887–895. [Google Scholar] [CrossRef]
- Mohapatra, A.; Viswabandya, A.; Samuel, R.; Deepti, A.N.; Madhivanan, S.; John, G.T. NK/T-Cell Lymphoma in a Renal Transplant Recipient and Review of Literature. Indian J. Nephrol. 2011, 21, 44–47. [Google Scholar] [CrossRef]
- Matsumura, M.; Mizuno, Y.; Okamoto, M.; Sawa, N.; Katayama, Y.; Shimoyama, N.; Kawagishi, N.; Miura, K. Long-Term Complete Remission of Multiple Extranodal Natural Killer/T-Cell-Type Posttransplant Lymphoproliferative Disorder after Surgical Resection: A Case Report. Transpl. Proc. 2014, 46, 2373–2376. [Google Scholar] [CrossRef] [PubMed]
- Gooptu, M.; Kim, H.T.; Chen, Y.-B.; Rybka, W.; Artz, A.; Boyer, M.; Johnston, L.; McGuirk, J.; Shea, T.C.; Jagasia, M.; et al. Effect of Antihuman T Lymphocyte Globulin on Immune Recovery after Myeloablative Allogeneic Stem Cell Transplantation with Matched Unrelated Donors: Analysis of Immune Reconstitution in a Double-Blind Randomized Controlled Trial. Biol. Blood Marrow Transpl. 2018, 24, 2216–2223. [Google Scholar] [CrossRef]
- Hadaya, K.; Avila, Y.; Valloton, L.; de Rham, C.; Bandelier, C.; Ferrari-Lacraz, S.; Pascual, M.; Pantaleo, G.; Martin, P.Y.; Buhler, L.; et al. Natural Killer Cell Receptor-Repertoire and Functions after Induction Therapy by Polyclonal Rabbit Anti-Thymocyte Globulin in Unsensitized Kidney Transplant Recipients. Clin. Immunol. 2010, 137, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Kho, M.M.L.; Bouvy, A.P.; Cadogan, M.; Kraaijeveld, R.; Baan, C.C.; Weimar, W. The Effect of Low and Ultra-Low Dosages Thymoglobulin on Peripheral T, B and NK Cells in Kidney Transplant Recipients. Transpl. Immunol. 2012, 26, 186–190. [Google Scholar] [CrossRef]
- Müller, T.F.; Grebe, S.O.; Neumann, M.C.; Heymanns, J.; Radsak, K.; Sprenger, H.; Lange, H. Persistent Long-Term Changes in Lymphocyte Subsets Induced by Polyclonal Antibodies. Transplantation 1997, 64, 1432–1437. [Google Scholar] [CrossRef]
- Savani, B.N.; Mielke, S.; Adams, S.; Uribe, M.; Rezvani, K.; Yong, A.S.M.; Zeilah, J.; Kurlander, R.; Srinivasan, R.; Childs, R.; et al. Rapid Natural Killer Cell Recovery Determines Outcome after T-Cell-Depleted HLA-Identical Stem Cell Transplantation in Patients with Myeloid Leukemias but Not with Acute Lymphoblastic Leukemia. Leukemia 2007, 21, 2145–2152. [Google Scholar] [CrossRef] [Green Version]
- Nakid-Cordero, C.; Choquet, S.; Gauthier, N.; Balegroune, N.; Tarantino, N.; Morel, V.; Arzouk, N.; Burrel, S.; Rousseau, G.; Charlotte, F.; et al. Distinct Immunopathological Mechanisms of EBV-positive and EBV-negative Posttransplant Lymphoproliferative Disorders. Am. J. Transplant. 2021, ajt.16547. [Google Scholar] [CrossRef]
- Baychelier, F.; Achour, A.; Nguyen, S.; Raphael, M.; Toubert, A.; Besson, C.; Arnoux, A.; Roos-Weil, D.; Marty, M.; Chapelier, A.; et al. Natural Killer Cell Deficiency in Patients with Non-Hodgkin Lymphoma after Lung Transplantation. J. Heart Lung Transpl. 2015, 34, 604–612. [Google Scholar] [CrossRef]
- Wiesmayr, S.; Webber, S.A.; Macedo, C.; Popescu, I.; Smith, L.; Luce, J.; Metes, D. Decreased NKp46 and NKG2D and Elevated PD-1 Are Associated with Altered NK-Cell Function in Pediatric Transplant Patients with PTLD. Eur. J. Immunol. 2012, 42, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Bergerson, R.J.; Williams, R.; Wang, H.; Shanley, R.; Colbenson, G.; Kerber, A.; Cooley, S.; Curtsinger, J.; Felices, M.; Miller, J.S.; et al. Fewer Circulating Natural Killer Cells 28 Days After Double Cord Blood Transplantation Predicts Inferior Survival and IL-15 Response. Blood Adv. 2016, 1, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Neudoerfl, C.; Mueller, B.J.; Blume, C.; Daemen, K.; Stevanovic-Meyer, M.; Keil, J.; Lehner, F.; Haller, H.; Falk, C.S. The Peripheral NK Cell Repertoire after Kidney Transplantation Is Modulated by Different Immunosuppressive Drugs. Front. Immunol. 2013, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peraldi, M.N.; Berrou, J.; Venot, M.; Chardiny, V.; Durrbach, A.; Vieillard, V.; Debré, P.; Charron, D.; Suberbielle, C.; Chevret, S.; et al. Natural Killer Lymphocytes Are Dysfunctional in Kidney Transplant Recipients on Diagnosis of Cancer. Transplantation 2015, 99, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Grzywacz, B.; Sukovich, D.; McCullar, V.; Cao, Q.; Lee, A.B.; Blazar, B.R.; Cornfield, D.N.; Miller, J.S.; Verneris, M.R. The Unexpected Effect of Cyclosporin A on CD56+CD16- and CD56+CD16+ Natural Killer Cell Subpopulations. Blood 2007, 110, 1530–1539. [Google Scholar] [CrossRef] [Green Version]
- González, J.P.; Zabaleta, A.; Sangro, P.; Basualdo, J.E.; Burgos, L.; Paiva, B.; Herrero, J.I. Immunophenotypic Pattern of De Novo Malignancy After Liver Transplantation. Transpl. Proc. 2019, 51, 77–79. [Google Scholar] [CrossRef]
- Sundström, Y.; Nilsson, C.; Lilja, G.; Kärre, K.; Troye-Blomberg, M.; Berg, L. The Expression of Human Natural Killer Cell Receptors in Early Life. Scand. J. Immunol. 2007, 66, 335–344. [Google Scholar] [CrossRef]
- Zhang, B.; Kracker, S.; Yasuda, T.; Casola, S.; Vanneman, M.; Hömig-Hölzel, C.; Wang, Z.; Derudder, E.; Li, S.; Chakraborty, T.; et al. Immune Surveillance and Therapy of Lymphomas Driven by Epstein-Barr Virus Protein LMP1 in a Mouse Model. Cell 2012, 148, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Nachmani, D.; Stern-Ginossar, N.; Sarid, R.; Mandelboim, O. Diverse Herpesvirus MicroRNAs Target the Stress-Induced Immune Ligand MICB to Escape Recognition by Natural Killer Cells. Cell Host Microbe 2009, 5, 376–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forconi, C.S.; Cosgrove, C.P.; Saikumar-Lakshmi, P.; Nixon, C.E.; Foley, J.; Ong’echa, J.M.; Otieno, J.A.; Alter, G.; Münz, C.; Moormann, A.M. Poorly Cytotoxic Terminally Differentiated CD56negCD16pos NK Cells Accumulate in Kenyan Children with Burkitt Lymphomas. Blood Adv. 2018, 2, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vély, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbé, C.; et al. PD-1 Mediates Functional Exhaustion of Activated NK Cells in Patients with Kaposi Sarcoma. Oncotarget 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front. Immunol. 2017, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a Subset of Human Natural Killer Cells Expressing High Levels of Programmed Death 1: A Phenotypic and Functional Characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakid-Cordero, C.; Arzouk, N.; Gauthier, N.; Tarantino, N.; Larsen, M.; Choquet, S.; Burrel, S.; Autran, B.; Vieillard, V.; Guihot, A. Skewed T Cell Responses to Epstein-Barr Virus in Long-Term Asymptomatic Kidney Transplant Recipients. PLoS ONE 2019, 14, e0224211. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cheng, Y.; Xu, Y.; Wang, Z.; Du, X.; Li, C.; Peng, J.; Gao, L.; Liang, X.; Ma, C. Increased Expression of Programmed Cell Death Protein 1 on NK Cells Inhibits NK-Cell-Mediated Anti-Tumor Function and Indicates Poor Prognosis in Digestive Cancers. Oncogene 2017, 36, 6143–6153. [Google Scholar] [CrossRef] [Green Version]
- Pittari, G.; Vago, L.; Festuccia, M.; Bonini, C.; Mudawi, D.; Giaccone, L.; Bruno, B. Restoring Natural Killer Cell Immunity against Multiple Myeloma in the Era of New Drugs. Front. Immunol. 2017, 8, 1444. [Google Scholar] [CrossRef] [Green Version]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Posttransplant Lymphoproliferative Disorders: Implications for Targeted Therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef] [Green Version]
- Kinch, A.; Sundström, C.; Baecklund, E.; Backlin, C.; Molin, D.; Enblad, G. Expression of PD-1, PD-L1, and PD-L2 in Posttransplant Lymphoproliferative Disorder after Solid Organ Transplantation. Leuk. Lymphoma 2018, 60, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Benson, D.M.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 Axis Modulates the Natural Killer Cell versus Multiple Myeloma Effect: A Therapeutic Target for CT-011, a Novel Monoclonal Anti–PD-1 Antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Galvez, C.; Anker, J.F.; Iams, W.T.; Bhave, M. Cancer Immunotherapy in a Neglected Population: The Current Use and Future of T-Cell-Mediated Checkpoint Inhibitors in Organ Transplant Patients. Cancer Treat. Rev. 2018, 63, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Zeitouni, N.; Fan, W.; Samie, F.H. Immune Checkpoint Inhibitor Therapy in Solid Organ Transplant Recipients: A Patient-Centered Systematic Review. J. Am. Acad. Dermatol. 2020, 82, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Nourse, J.P.; Morrison, L.; Nguyen-Van, D.; Moss, D.J.; Burrows, S.R.; Gandhi, M.K. Expansion of EBNA1-Specific Effector T Cells in Posttransplantation Lymphoproliferative Disorders. Blood 2010, 116, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Calarota, S.A.; Chiesa, A.; Zelini, P.; Comolli, G.; Minoli, L.; Baldanti, F. Detection of Epstein-Barr Virus-Specific Memory CD4+ T Cells Using a Peptide-Based Cultured Enzyme-Linked Immunospot Assay. Immunology 2013, 139, 533–544. [Google Scholar] [CrossRef]
- Morscio, J.; Dierickx, D.; Ferreiro, J.F.; Herreman, A.; Van Loo, P.; Bittoun, E.; Verhoef, G.; Matthys, P.; Cools, J.; Wlodarska, I.; et al. Gene Expression Profiling Reveals Clear Differences Between EBV-Positive and EBV-Negative Posttransplant Lymphoproliferative Disorders. Am. J. Transpl. 2013, 13, 1305–1316. [Google Scholar] [CrossRef]
- Jog, N.R.; Chakravarty, E.F.; Guthridge, J.M.; James, J.A. Epstein Barr Virus Interleukin 10 Suppresses Anti-Inflammatory Phenotype in Human Monocytes. Front. Immunol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Muti, G.; Klersy, C.; Baldanti, F.; Granata, S.; Oreste, P.; Pezzetti, L.; Gatti, M.; Gargantini, L.; Caramella, M.; Mancini, V. Epstein-Barr Virus (EBV) Load and Interleukin-10 in Lymphoproliferative Disorders. Br. J. Haematol. 2003, 122, 927–933. [Google Scholar] [CrossRef]
- Marcelis, L.; Tousseyn, T. The Tumor Microenvironment in Post-Transplant Lymphoproliferative Disorders. Cancer Microenviron. 2019. [Google Scholar] [CrossRef]
- Quinlan, S.C.; Pfeiffer, R.M.; Morton, L.M.; Engels, E.A. Risk Factors for Early-Onset and Late-Onset Post-Transplant Lymphoproliferative Disorder in Kidney Recipients in the United States. Am. J. Hematol. 2011, 86, 206–209. [Google Scholar] [CrossRef] [Green Version]
- Naik, S.; Riches, M.; Hari, P.; Kim, S.; Chen, M.; Bachier, C.; Shaughnessy, P.; Hill, J.; Ljungman, P.; Battiwalla, M.; et al. Survival Outcomes of Allogeneic Hematopoietic Cell Transplants with EBV-positive or EBV-negative Post-transplant Lymphoproliferative Disorder, A CIBMTR Study. Transpl. Infect. Dis. 2019, 21, e13145. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, D.; Tousseyn, T.; Sagaert, X.; Fieuws, S.; Wlodarska, I.; Morscio, J.; Brepoels, L.; Kuypers, D.; Vanhaecke, J.; Nevens, F.; et al. Single-Center Analysis of Biopsy-Confirmed Posttransplant Lymphoproliferative Disorder: Incidence, Clinicopathological Characteristics and Prognostic Factors. Leuk. Lymphoma 2013, 54, 2433–2440. [Google Scholar] [CrossRef]
- Bishnoi, R.; Minish, J.; Franke, A.J.; Skelton, W.P.; Shah, C.P.; Wang, Y.; Dang, N.H. Single-Institution Retrospective Analysis of Prognostic Factors Influencing Very Late-Onset Post-Transplant Lymphoproliferative Disorder. Cureus 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Ndhlovu, L.C.; Lopez-Verge, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 Marks Human Natural Killer Cell Maturation and Suppresses Cell-Mediated Cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves Silva, I.; Yasinska, I.M.; Sakhnevych, S.S.; Fiedler, W.; Wellbrock, J.; Bardelli, M.; Varani, L.; Hussain, R.; Siligardi, G.; Ceccone, G.; et al. The Tim-3-Galectin-9 Secretory Pathway Is Involved in the Immune Escape of Human Acute Myeloid Leukemia Cells. EBioMedicine 2017, 22, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Tallerico, R.; Cristiani, C.M.; Staaf, E.; Garofalo, C.; Sottile, R.; Capone, M.; Pico de Coaña, Y.; Madonna, G.; Palella, E.; Wolodarski, M.; et al. IL-15, TIM-3 and NK Cells Subsets Predict Responsiveness to Anti-CTLA-4 Treatment in Melanoma Patients. OncoImmunology 2017, 6, e1261242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadadi, L.; Hafezi, M.; Amirzargar, A.A.; Sharifian, R.A.; Abediankenari, S.; Asgarian-Omran, H. Dysregulated Expression of Tim-3 and NKp30 Receptors on NK Cells of Patients with Chronic Lymphocytic Leukemia. Oncol. Res. Treat. 2019, 42, 197–203. [Google Scholar] [CrossRef] [PubMed]
- So, E.C.; Khaladj-Ghom, A.; Ji, Y.; Amin, J.; Song, Y.; Burch, E.; Zhou, H.; Sun, H.; Chen, S.; Bentzen, S.; et al. NK Cell Expression of Tim-3: First Impressions Matter. Immunobiology 2019, 224, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, Y.; Tan, L.; Yu, W.; Chen, D.; Lu, C.; He, J.; Wu, G.; Liu, X.; Zhang, Y. Increased Tim-3 Expression in Peripheral NK Cells Predicts a Poorer Prognosis and Tim-3 Blockade Improves NK Cell-Mediated Cytotoxicity in Human Lung Adenocarcinoma. Int. Immunopharmacol. 2015, 29, 635–641. [Google Scholar] [CrossRef]
- Weng, W.-K.; Levy, R. Two Immunoglobulin G Fragment C Receptor Polymorphisms Independently Predict Response to Rituximab in Patients with Follicular Lymphoma. J. Clin. Oncol. 2003, 21, 3940–3947. [Google Scholar] [CrossRef]
- Cartron, G.; Dacheux, L.; Salles, G.; Solal-Celigny, P.; Bardos, P.; Colombat, P.; Watier, H. Therapeutic Activity of Humanized Anti-CD20 Monoclonal Antibody and Polymorphism in IgG Fc Receptor FcgammaRIIIa Gene. Blood 2002, 99, 754–758. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.-K.; Negrin, R.S.; Lavori, P.; Horning, S.J. Immunoglobulin G Fc Receptor FcgammaRIIIa 158 V/F Polymorphism Correlates with Rituximab-Induced Neutropenia after Autologous Transplantation in Patients with Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2010, 28, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, R.L.; Namenuk, A.K.; Hong, K.; Meng, Y.G.; Rae, J.; Briggs, J.; Xie, D.; Lai, J.; Stadlen, A.; Li, B.; et al. High Resolution Mapping of the Binding Site on Human IgG1 for Fc Gamma RI, Fc Gamma RII, Fc Gamma RIII, and FcRn and Design of IgG1 Variants with Improved Binding to the Fc Gamma R. J. Biol. Chem. 2001, 276, 6591–6604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, W.-K.; Czerwinski, D.; Timmerman, J.; Hsu, F.J.; Levy, R. Clinical Outcome of Lymphoma Patients after Idiotype Vaccination Is Correlated with Humoral Immune Response and Immunoglobulin G Fc Receptor Genotype. J. Clin. Oncol. 2004, 22, 4717–4724. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.-K.; Weng, W.-K.; Levy, R. Immunoglobulin G Fc Receptor Polymorphisms Do Not Correlate with Response to Chemotherapy or Clinical Course in Patients with Follicular Lymphoma. Leuk. Lymphoma 2009, 50, 1494–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, A.; Ayello, J.; Van de Ven, C.; Elmacken, M.; Sabulski, A.; Barth, M.J.; Czuczman, M.S.; Islam, H.; Klein, C.; Cairo, M.S. Obinutuzumab (GA101) Compared to Rituximab Significantly Enhances Cell Death and Antibody-Dependent Cytotoxicity and Improves Overall Survival against CD20(+) Rituximab-Sensitive/-Resistant Burkitt Lymphoma (BL) and Precursor B-Acute Lymphoblastic Leukaemia (Pre-B-ALL): Potential Targeted Therapy in Patients with Poor Risk CD20(+) BL and Pre-B-ALL. Br. J. Haematol. 2015, 171, 763–775. [Google Scholar] [CrossRef]
- Schmohl, J.U.; Gleason, M.K.; Dougherty, P.R.; Miller, J.S.; Vallera, D.A. Heterodimeric Bispecific Single Chain Variable Fragments (ScFv) Killer Engagers (BiKEs) Enhance NK-Cell Activity Against CD133+ Colorectal Cancer Cells. Target. Oncol. 2016, 11, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Wiernik, A.; Foley, B.; Zhang, B.; Verneris, M.R.; Warlick, E.; Gleason, M.K.; Ross, J.A.; Luo, X.; Weisdorf, D.J.; Walcheck, B.; et al. Targeting Natural Killer Cells to Acute Myeloid Leukemia in Vitro with a CD16 x 33 Bispecific Killer Cell Engager and ADAM17 Inhibition. Clin. Cancer Res. 2013, 19, 3844–3855. [Google Scholar] [CrossRef] [Green Version]
- Gleason, M.K.; Ross, J.A.; Warlick, E.D.; Lund, T.C.; Verneris, M.R.; Wiernik, A.; Spellman, S.; Haagenson, M.D.; Lenvik, A.J.; Litzow, M.R.; et al. CD16xCD33 Bispecific Killer Cell Engager (BiKE) Activates NK Cells against Primary MDS and MDSC CD33+ Targets. Blood 2014, 123, 3016–3026. [Google Scholar] [CrossRef]
- Vallera, D.A.; Zhang, B.; Gleason, M.K.; Oh, S.; Weiner, L.M.; Kaufman, D.S.; McCullar, V.; Miller, J.S.; Verneris, M.R. Heterodimeric Bispecific Single-Chain Variable-Fragment Antibodies against EpCAM and CD16 Induce Effective Antibody-Dependent Cellular Cytotoxicity against Human Carcinoma Cells. Cancer Biother. Radiopharm. 2013, 28, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, A.; Nagai, K.; Nakanishi, T.; Kumagai, I.; Asano, R. Functional Domain Order of an Anti-EGFR × Anti-CD16 Bispecific Diabody Involving NK Cell Activation. Int. J. Mol. Sci. 2020, 21, 8914. [Google Scholar] [CrossRef]
- Thakur, A.; Huang, M.; Lum, L.G. Bispecific Antibody Based Therapeutics: Strengths and Challenges. Blood Rev. 2018, 32, 339–347. [Google Scholar] [CrossRef]
- Schmohl, J.U.; Felices, M.; Taras, E.; Miller, J.S.; Vallera, D.A. Enhanced ADCC and NK Cell Activation of an Anticarcinoma Bispecific Antibody by Genetic Insertion of a Modified IL-15 Cross-Linker. Mol. Ther. 2016, 24, 1312–1322. [Google Scholar] [CrossRef] [Green Version]
- Schmohl, J.U.; Felices, M.; Todhunter, D.; Taras, E.; Miller, J.S.; Vallera, D.A. Tetraspecific ScFv Construct Provides NK Cell Mediated ADCC and Self-Sustaining Stimuli via Insertion of IL-15 as a Cross-Linker. Oncotarget 2016, 7, 73830–73844. [Google Scholar] [CrossRef]
- Cheng, Y.; Zheng, X.; Wang, X.; Chen, Y.; Wei, H.; Sun, R.; Tian, Z.; Sun, H. Trispecific Killer Engager 161519 Enhances Natural Killer Cell Function and Provides Anti-Tumor Activity against CD19-Positive Cancers. Cancer Biol. Med. 2020, 17, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Rothe, A.; Sasse, S.; Topp, M.S.; Eichenauer, D.A.; Hummel, H.; Reiners, K.S.; Dietlein, M.; Kuhnert, G.; Kessler, J.; Buerkle, C.; et al. A Phase 1 Study of the Bispecific Anti-CD30/CD16A Antibody Construct AFM13 in Patients with Relapsed or Refractory Hodgkin Lymphoma. Blood 2015, 125, 4024–4031. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, N.L.; Herrera, A.F.; Domingo-Domenech, E.; Mehta, A.; Forero-Torres, A.; Garcia-Sanz, R.; Armand, P.; Devata, S.; Izquierdo, A.R.; Lossos, I.S.; et al. A Phase 1b Study of AFM13 in Combination with Pembrolizumab in Patients with Relapsed or Refractory Hodgkin Lymphoma. Blood 2020, 136, 2401–2409. [Google Scholar] [CrossRef]
- Felices, M.; Kodal, B.; Hinderlie, P.; Kaminski, M.F.; Cooley, S.; Weisdorf, D.J.; Vallera, D.A.; Miller, J.S.; Bachanova, V. Novel CD19-Targeted TriKE Restores NK Cell Function and Proliferative Capacity in CLL. Blood Adv. 2019, 3, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713. [Google Scholar] [CrossRef]
- Burns, L.J.; Weisdorf, D.J.; DeFor, T.E.; Vesole, D.H.; Repka, T.L.; Blazar, B.R.; Burger, S.R.; Panoskaltsis-Mortari, A.; Keever-Taylor, C.A.; Zhang, M.-J.; et al. IL-2-Based Immunotherapy after Autologous Transplantation for Lymphoma and Breast Cancer Induces Immune Activation and Cytokine Release: A Phase I/II Trial. Bone Marrow Transpl. 2003, 32, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.A. Interleukin-2: Inception, Impact, and Implications. Science 1988, 240, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, Hyperproliferation, Activation of Natural Killer Cells and CD8 T Cells, and Cytokine Production during First-in-Human Clinical Trial of Recombinant Human Interleukin-15 in Patients with Cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.E.K.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C.; et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (RhIL15) in Adults with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1525–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romee, R.; Cooley, S.; Berrien-Elliott, M.M.; Westervelt, P.; Verneris, M.R.; Wagner, J.E.; Weisdorf, D.J.; Blazar, B.R.; Ustun, C.; DeFor, T.E.; et al. First-in-Human Phase 1 Clinical Study of the IL-15 Superagonist Complex ALT-803 to Treat Relapse after Transplantation. Blood 2018, 131, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Hess, B.T.; Bachanova, V.; Becker-Hapak, M.; McClain, E.; Berrien-Elliott, M.; Wagner, J.; Bartlett, N.L.; Kahl, B.; Mehta-Shah, N.; et al. Abstract CT146: First-in-Human Phase I Combination of the IL-15 Receptor Super Agonist Complex ALT-803 with a Therapeutic (Anti-CD20) Monoclonal Antibody (MAb) for Patients with Relapsed or Refractory Indolent Non-Hodgkin Lymphoma (INHL). Cancer Res. 2018, 78, CT146. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Riley, J.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive Transfer of Autologous Natural Killer Cells Leads to High Levels of Circulating Natural Killer Cells but Does Not Mediate Tumor Regression. Clin. Cancer Res. 2011, 17, 6287–6297. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Leitman, S.; Chang, A.E.; Ettinghausen, S.E.; Matory, Y.L.; Skibber, J.M.; Shiloni, E.; Vetto, J.T. Observations on the Systemic Administration of Autologous Lymphokine-Activated Killer Cells and Recombinant Interleukin-2 to Patients with Metastatic Cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P.; Yang, J.C.; Morgan, R.A.; Dudley, M.E. Adoptive Cell Transfer: A Clinical Path to Effective Cancer Immunotherapy. Nat. Rev. Cancer 2008, 8, 299–308. [Google Scholar] [CrossRef]
- Bachanova, V.; Cooley, S.; Defor, T.E.; Verneris, M.R.; Zhang, B.; McKenna, D.H.; Curtsinger, J.; Panoskaltsis-Mortari, A.; Lewis, D.; Hippen, K.; et al. Clearance of Acute Myeloid Leukemia by Haploidentical Natural Killer Cells Is Improved Using IL-2 Diphtheria Toxin Fusion Protein. Blood 2014, 123, 3855–3863. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical NK Cells in Patients with Cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [Green Version]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.M.; DeFor, T.E.; Curtsinger, J.M.; Robertson, P.; Grzywacz, B.; Conlon, K.C.; Waldmann, T.A.; et al. First-in-Human Trial of RhIL-15 and Haploidentical Natural Killer Cell Therapy for Advanced Acute Myeloid Leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Sarhan, D.; DeFor, T.E.; Cooley, S.; Panoskaltsis-Mortari, A.; Blazar, B.R.; Curtsinger, J.M.; Burns, L.; Weisdorf, D.J.; Miller, J.S. Haploidentical Natural Killer Cells Induce Remissions in Non-Hodgkin Lymphoma Patients with Low Levels of Immune-Suppressor Cells. Cancer Immunol. Immunother. 2018, 67, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Berrien-Elliott, M.M.; Wagner, J.A.; Fehniger, T.A. Human Cytokine-Induced Memory-Like Natural Killer Cells. J. Innate Immun. 2015, 7, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-Induced Memory-like Natural Killer Cells Exhibit Enhanced Responses against Myeloid Leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef] [Green Version]
- Bachanova, V.; Maakaron, J.; McKenna, D.H.; Cao, Q.; DeFor, T.E.; He, F.; Janakiram, M.; Wangen, R.; Cayci, Z.; Grzywacz, B.; et al. Results of a Phase 1 Trial of Gda-201, Nicotinamide-Expanded Allogeneic Natural Killer (NK) Cells in Patients with Refractory Non-Hodgkin Lymphoma (NHL) and Multiple Myeloma. Blood 2020, 136, 6. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Gang, M.; Marin, N.D.; Wong, P.; Neal, C.C.; Marsala, L.; Foster, M.; Schappe, T.; Meng, W.; Tran, J.; Schaettler, M.; et al. CAR-Modified Memory-like NK Cells Exhibit Potent Responses to NK-Resistant Lymphomas. Blood 2020, 136, 2308–2318. [Google Scholar] [CrossRef] [PubMed]

| WHO Classification | Post-Transplant Onset | Age at Transplant | EBV Association |
|---|---|---|---|
| Nondestructive PTLD | Generally early | Adult and pediatric | Generally EBV-positive |
| Polymorphic PTLD | Frequently early | Adult and pediatric | Generally EBV-positive |
| Monomorphic PTLD | |||
| B-cell lymphomas | Both early and late | More frequent in adult than pediatric | EBV-positive and EBV-negative |
| T-cell and NK-cell lymphomas | Generally late | Generally adult | Frequently EBV-negative |
| Classic Hodgkin Lymphoma-like PTLD | Generally late | Generally adult | Frequently EBV-positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakid-Cordero, C.; Baron, M.; Guihot, A.; Vieillard, V. Natural Killer Cells in Post-Transplant Lymphoproliferative Disorders. Cancers 2021, 13, 1836. https://doi.org/10.3390/cancers13081836
Nakid-Cordero C, Baron M, Guihot A, Vieillard V. Natural Killer Cells in Post-Transplant Lymphoproliferative Disorders. Cancers. 2021; 13(8):1836. https://doi.org/10.3390/cancers13081836
Chicago/Turabian StyleNakid-Cordero, Cecilia, Marine Baron, Amélie Guihot, and Vincent Vieillard. 2021. "Natural Killer Cells in Post-Transplant Lymphoproliferative Disorders" Cancers 13, no. 8: 1836. https://doi.org/10.3390/cancers13081836