Transmembrane Activator and CAML Interactor (TACI): Another Potential Target for Immunotherapy of Multiple Myeloma?
Abstract
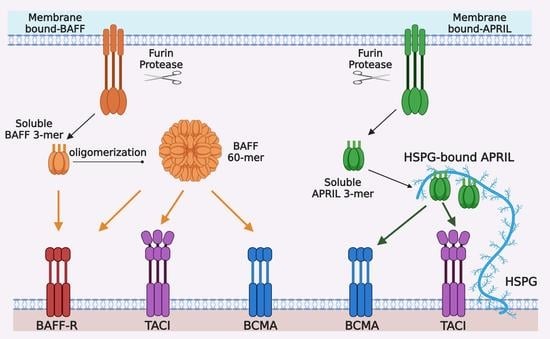
:1. Introduction
2. The Role of TACI in Normal B Cells and MM
2.1. TACI is Important for Regulating B Cell Functions
2.2. TACI is Important for the Differentiation and Survival of Plasma Cells
2.3. TACI in the Pathophysiology of MM
3. Targeting TACI in Immunotherapy Against MM
3.1. Soluble TACI-Fc Fusion Protein Inhibits Myeloma Cell Growth and Survival
3.2. Antagonistic Anti-APRIL Antibody Inhibits MM Growth and Modulates Immunosuppressive BM Microenvironment
3.3. Incorporating TACI in CAR-T Cells Targeting of MM
4. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Wilcock, P.; Webster, R. The multiple myeloma drug market. Nat. Rev. Drug Discov. 2019, 18, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; Van Duin, M.; Sonneveld, P.; Mateos, M.-V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Prim. 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Brill, I.K.; Omel, J.; Godby, K.; Kumar, S.K.; Brown, E.E. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017, 1, 282–287. [Google Scholar] [CrossRef]
- D’Agostino, M.; Boccadoro, M.; Smith, E. Novel Immunotherapies for Multiple Myeloma. Curr. Hematol. Malign-Rep. 2017, 12, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2017, 32, 252–262. [Google Scholar] [CrossRef]
- Shay, G.; Hazlehurst, L.; Lynch, C. Dissecting the multiple myeloma-bone microenvironment reveals new therapeutic opportunities. J. Mol. Med. 2015, 94, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Mirandola, L.; Platonova, N.; Apicella, L.; Basile, A.; Figueroa, A.J.; Cobos, E.; Chiriva-Internati, M.; Chiaramonte, R. Notch-directed microenvironment reprogramming in myeloma: A single path to multiple outcomes. Leukemia 2013, 27, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Mitsiades, C.S.; Mitsiades, N.S.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. The role of the bone microenvironment in the pathophysiology and therapeutic management of multiple myeloma: Interplay of growth factors, their receptors and stromal interactions. Eur. J. Cancer 2006, 42, 1564–1573. [Google Scholar] [CrossRef]
- Pagnucco, G.; Cardinale, G.; Gervasi, F. Targeting Multiple Myeloma Cells and Their Bone Marrow Microenvironment. Ann. N. Y. Acad. Sci. 2004, 1028, 390–399. [Google Scholar] [CrossRef]
- Moreaux, J.; Legouffe, E.; Jourdan, E.; Quittet, P.; Rème, T.; Lugagne, C.; Moine, P.; Rossi, J.-F.; Klein, B.; Tarte, K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004, 103, 3148–3157. [Google Scholar] [CrossRef] [Green Version]
- Novak, A.J.; Darce, J.R.; Arendt, B.K.; Harder, B.; Henderson, K.; Kindsvogel, W.; Gross, J.A.; Greipp, P.R.; Jelinek, D.F. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A mechanism for growth and survival. Blood 2004, 103, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, Y.-T. Role of B-Cell-Activating Factor in Adhesion and Growth of Human Multiple Myeloma Cells in the Bone Marrow Microenvironment. Cancer Res. 2006, 66, 6675–6682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, M.; Kido, S.; Hiasa, M.; Nakano, A.; Oda, A.; Amou, H.; Matsumoto, T. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: A rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia 2006, 20, 1313–1315. [Google Scholar] [CrossRef] [Green Version]
- Schneider, P.; Mackay, F.; Steiner, V.; Hofmann, K.; Bodmer, J.-L.; Holler, N.; Ambrose, C.; Lawton, P.; Bixler, S.; Acha-Orbea, H.; et al. BAFF, a Novel Ligand of the Tumor Necrosis Factor Family, Stimulates B Cell Growth. J. Exp. Med. 1999, 189, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.A.; Johnston, J.; Mudri, S.; Enselman, R.; Dillon, S.R.; Madden, K.; Xu, W.; Parrish-Novak, J.; Foster, D.; Lofton-Day, C.; et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 2000, 404, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A.; Belvedere, O.; Orr, A.; Pieri, K.; LaFleur, D.W.; Feng, P.; Soppet, D.; Charters, M.; Gentz, R.; Parmelee, D.; et al. BLyS: Member of the Tumor Necrosis Factor Family and B Lymphocyte Stimulator. Science 1999, 285, 260–263. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Ni, J.; Zhai, Y.; Yu, G.L.; Aggarwal, B.B. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J. Boil. Chem. 1999, 274, 15978–15981. [Google Scholar] [CrossRef] [Green Version]
- Shu, H.-B.; Hu, W.-H.; Johnson, H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J. Leukoc. Boil. 1999, 65, 680–683. [Google Scholar] [CrossRef]
- Hahne, M.; Kataoka, T.; Schröter, M.; Hofmann, K.; Irmler, M.; Bodmer, J.-L.; Schneider, P.; Bornand, T.; Holler, N.; French, L.; et al. APRIL, a New Ligand of the Tumor Necrosis Factor Family, Stimulates Tumor Cell Growth. J. Exp. Med. 1998, 188, 1185–1190. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.; Manos, E.; Jensen, G.; Nadauld, L.; Jones, D.A. APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res. 2000, 60, 1021–1027. [Google Scholar]
- Rennert, P.; Schneider, P.; Cachero, T.G.; Thompson, J.; Trabach, L.; Hertig, S.; Holler, N.; Qian, F.; Mullen, C.; Strauch, K.; et al. A Soluble Form of B Cell Maturation Antigen, a Receptor for the Tumor Necrosis Factor Family Member April, Inhibits Tumor Cell Growth. J. Exp. Med. 2000, 192, 1677–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsters, S.A.; Yan, M.; Pitti, R.M.; E Haas, P.; Dixit, V.M.; Ashkenazi, A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr. Boil. 2000, 10, 785–788. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Bressette, D.; Carrell, J.A.; Kaufman, T.; Feng, P.; Taylor, K.; Gan, Y.; Cho, Y.H.; Garcia, A.D.; Gollatz, E.; et al. Tumor Necrosis Factor (TNF) Receptor Superfamily Member TACI Is a High Affinity Receptor for TNF Family Members APRIL and BLyS. J. Boil. Chem. 2000, 275, 35478–35485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.-Z.; Treanor, J.; Senaldi, G.; Khare, S.D.; Boone, T.; Kelley, M.; Theill, L.E.; Colombero, A.; Solovyev, I.; Lee, F.; et al. Taci Is a Traf-Interacting Receptor for Tall-1, a Tumor Necrosis Factor Family Member Involved in B Cell Regulation. J. Exp. Med. 2000, 192, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Boone, T.; Delaney, J.; Hawkins, N.; Kelley, M.; Ramakrishnan, M.; McCabe, S.; Qiu, W.-R.; Kornuc, M.; Xia, X.-Z.; et al. APRIL and TALL-1 and receptors BCMA and TACI: System for regulating humoral immunity. Nat. Immunol. 2000, 1, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.S.; Schneider, P.; Kalled, S.L.; Wang, L.; Lefevre, E.A.; Cachero, T.G.; Mackay, F.; Bixler, S.A.; Zafari, M.; Liu, Z.-Y.; et al. Baff Binds to the Tumor Necrosis Factor Receptor–Like Molecule B Cell Maturation Antigen and Is Important for Maintaining the Peripheral B Cell Population. J. Exp. Med. 2000, 192, 129–136. [Google Scholar] [CrossRef]
- Thompson, J.S.; Bixler, S.A.; Qian, F.; Vora, K.; Scott, M.L.; Cachero, T.G.; Hession, C.; Schneider, P.; Sizing, I.D.; Mullen, C.; et al. BAFF-R, a Newly Identified TNF Receptor That Specifically Interacts with BAFF. Science 2001, 293, 2108–2111. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Brady, J.R.; Chan, B.; Lee, W.P.; Hsu, B.; Harless, S.; Cancro, M.P.; Grewal, I.S.; Dixit, V.M. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr. Boil. 2001, 11, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, J.; Planelles, L.; De Jong-Odding, J.; Hardenberg, G.; Pals, S.T.; Hahne, M.; Spaargaren, M.; Medema, J.P. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005, 12, 637–648. [Google Scholar] [CrossRef] [Green Version]
- Ingold, K.; Zumsteg, A.; Tardivel, A.; Huard, B.; Steiner, Q.-G.; Cachero, T.G.; Qiang, F.; Gorelik, L.; Kalled, S.L.; Acha-Orbea, H.; et al. Identification of proteoglycans as the APRIL-specific binding partners. J. Exp. Med. 2005, 201, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia 2020, 34, 985–1005. [Google Scholar] [CrossRef] [PubMed]
- Roex, G.; Feys, T.; Beguin, Y.; Kerre, T.; Poiré, X.; Lewalle, P.; Vandenberghe, P.; Bron, D.; Anguille, S. Chimeric Antigen Receptor-T-Cell Therapy for B-Cell Hematological Malignancies: An Update of the Pivotal Clinical Trial Data. Pharmaceutics 2020, 12, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Amill, L.; Suñe, G.; Antoñana-Vildosola, A.; Castella, M.; Najjar, A.; Bonet, J.; Fernández-Fuentes, N.; Inogés, S.; López, A.; Bueno, C.; et al. Preclinical development of a humanized chimeric antigen receptor against B cell maturation antigen for multiple myeloma. Haematologica 2020. [Google Scholar] [CrossRef] [PubMed]
- Von Bülow, G.-U.; Bram, R.J. NF-AT Activation Induced by a CAML-Interacting Member of the Tumor Necrosis Factor Receptor Superfamily. Science 1997, 278, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Von Bülow, G.-U.; Russell, H.; Copeland, N.; Gilbert, D.J.; Jenkins, N.A.; Bram, R.J. Molecular cloning and functional characterization of murine transmembrane activator and CAML interactor (TACI) with chromosomal localization in human and mouse. Mamm. Genome 2000, 11, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Von Bülow, G.-U.; Van Deursen, J.M.; Bram, R.J. Regulation of the T-Independent Humoral Response by TACI. Immunity 2001, 14, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Wang, H.; Chan, B.; Roose-Girma, M.; Erickson, S.; Baker, T.; Tumas, D.; Grewal, I.S.; Dixit, V.M. Activation and accumulation of B cells in TACI-deficient mice. Nat. Immunol. 2001, 2, 638–643. [Google Scholar] [CrossRef]
- Gross, J.A.; Dillon, S.R.; McMillen, S.; Waggie, K.; Schreckhise, R.W.; Shoemaker, K.; Vu, T.; Moore, M.; Grossman, A.; Clegg, C.H.; et al. TACI-Ig neutralizes molecules critical for b cell development and autoimmune disease: Impaired b cell maturation in mice lacking BLyS. Immunity 2001, 15, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Castigli, E.; Wilson, S.A.; Scott, S.; Dedeoglu, F.; Xu, S.; Lam, K.-P.; Bram, R.J.; Jabara, H.; Geha, R.S. TACI and BAFF-R mediate isotype switching in B cells. J. Exp. Med. 2005, 201, 35–39. [Google Scholar] [CrossRef] [Green Version]
- Castigli, E.; Wilson, S.A.; Garibyan, L.; Rachid, R.; Bonilla, F.; Schneider, L.; Geha, R.S. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat. Genet. 2005, 37, 829–834. [Google Scholar] [CrossRef]
- Salzer, U.; Chapel, H.M.; Webster, A.D.B.; Pan-Hammarstrom, Q.; Schmitt-Graeff, A.; Schlesier, M.; Peter, H.H.; Rockstroh, J.K.; Schneider, P.; Schäffer, A.A.; et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat. Genet. 2005, 37, 820–828. [Google Scholar] [CrossRef]
- Castigli, E.; Wilson, S.A.; Elkhal, A.; Ozcan, E.; Garibyan, L.; Geha, R.S. Transmembrane activator and calcium modulator and cyclophilin ligand interactor enhances CD40-driven plasma cell differentiation. J. Allergy Clin. Immunol. 2007, 120, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Mantchev, G.T.; Cortesão, C.S.; Rebrovich, M.; Cascalho, M.; Bram, R.J. TACI Is Required for Efficient Plasma Cell Differentiation in Response to T-Independent Type 2 Antigens. J. Immunol. 2007, 179, 2282–2288. [Google Scholar] [CrossRef] [Green Version]
- Romberg, N.; Chamberlain, N.; Saadoun, D.; Gentile, M.; Kinnunen, T.; Ng, Y.S.; Virdee, M.; Menard, L.; Cantaert, T.; Morbach, H.; et al. CVID-associated TACI mutations affect autoreactive B cell selection and activation. J. Clin. Investig. 2013, 123, 4283–4293. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, E.; Garibyan, L.; Lee, J.J.-Y.; Bram, R.J.; Lam, K.-P.; Geha, R.S. Transmembrane activator, calcium modulator, and cyclophilin ligand interactor drives plasma cell differentiation in LPS-activated B cells. J. Allergy Clin. Immunol. 2009, 123, 1277–1286.e5. [Google Scholar] [CrossRef]
- He, B.; Santamaria, R.; Xu, W.; Cols, M.; Chen, K.; Puga, I.; Shan, M.; Xiong, H.; Bussel, J.B.; Chiu, A.; et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 2010, 11, 836–845. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, S.; Cortesão, C.; Bram, R.J.; Platt, J.L.; Cascalho, M. TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood 2011, 118, 5832–5839. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Xu, S.; Lam, K.-P. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc. Natl. Acad. Sci. USA 2012, 109, 15401–15406. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Carmona, Y.; Cols, M.; Ting, A.T.; Radigan, L.; Yuk, F.J.; Zhang, L.; Cerutti, A.; Cunningham-Rundles, C. Differential induction of plasma cells by isoforms of human TACI. Blood 2015, 125, 1749–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saulep-Easton, D.; Vincent, F.B.; Quah, P.S.; Wei, A.; Ting, S.B.; Croce, C.M.; Tam, C.S.; Mackay, F. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia 2016, 30, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.-T.; Lin, L.; Xing, L.; Cho, S.-F.; Yu, T.; Acharya, C.; Wen, K.; Hsieh, P.A.; Dulos, J.; Van Elsas, A.; et al. APRIL signaling via TACI mediates immunosuppression by T regulatory cells in multiple myeloma: Therapeutic implications. Leukemia 2019, 33, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lam, K.-P. B-Cell Maturation Protein, Which Binds the Tumor Necrosis Factor Family Members BAFF and APRIL, Is Dispensable for Humoral Immune Responses. Mol. Cell. Boil. 2001, 21, 4067–4074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiemann, B.; Tudhope, A.W.; Chilcott, C.P.; McCulloch, M.; Cook, E.R.; Chappell, J.; Ellam, R.M.; Lea, D.W.; Lough, J.M.; Shimmield, G.B. An Essential Role for BAFF in the Normal Development of B Cells Through a BCMA-Independent Pathway. Science 2001, 293, 2111–2114. [Google Scholar] [CrossRef]
- Sasaki, Y.; Casola, S.; Kutok, J.L.; Rajewsky, K.; Schmidt-Supprian, M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J. Immunol. 2004, 173, 2245–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garibyan, L.; Lobito, A.A.; Siegel, R.M.; Call, M.; Wucherpfennig, K.W.; Geha, R.S. Dominant-negative effect of the heterozygous C104R TACI mutation in common variable immunodeficiency (CVID). J. Clin. Investig. 2007, 117, 1550–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.J.; Jabara, H.H.; Garibyan, L.; Rauter, I.; Sannikova, T.; Dillon, S.R.; Bram, R.; Geha, R.S. The C104R mutant impairs the function of transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) through haploinsufficiency. J. Allergy Clin. Immunol. 2010, 126, 1234–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinen, J.; Martinez-Gallo, M.; Gu, W.; Cols, M.; Cerutti, A.; Radigan, L.; Zhang, L.; Potocki, L.; Withers, M.; Lupski, J.R.; et al. Transmembrane activator and CAML interactor (TACI) haploinsufficiency results in B-cell dysfunction in patients with Smith-Magenis syndrome. J. Allergy Clin. Immunol. 2011, 127, 1579–1586.e2. [Google Scholar] [CrossRef] [Green Version]
- Salzer, U.; Bacchelli, C.; Buckridge, S.; Pan-Hammarstrom, Q.; Jennings, S.; Lougaris, V.; Bergbreiter, A.; Hagena, T.; Birmelin, J.; Plebani, A.; et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood 2009, 113, 1967–1976. [Google Scholar] [CrossRef] [Green Version]
- Bossen, C.; Cachero, T.G.; Tardivel, A.; Ingold, K.; Willen, L.; Dobles, M.; Scott, M.L.; Maquelin, A.; Belnoue, E.; Siegrist, C.-A.; et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood 2008, 111, 1004–1012. [Google Scholar] [CrossRef] [Green Version]
- Jourdan, M.; Zhang, X.G.; Portier, M.; Boiron, J.M.; Bataille, R.; Klein, B. IFN-alpha induces autocrine production of IL-6 in myeloma cell lines. J. Immunol. 1991, 147, 4402–4407. [Google Scholar]
- Jelinek, D.F.; Witzig, T.E.; Arendt, B.K. A role for insulin-like growth factor in the regulation of IL-6-responsive human myeloma cell line growth. J. Immunol. 1997, 159, 487–496. [Google Scholar]
- Derksen, P.W.B.; Keehnen, R.M.J.; Evers, L.M.; Van Oers, M.H.J.; Spaargaren, M.; Pals, S.T. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma. Blood 2002, 99, 1405–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.D.; De Vos, J.; Jourdan, M.; Couderc, G.; Lu, Z.-Y.; Rossi, J.-F.; Klein, B. Cooperation between heparin-binding EGF-like growth factor and interleukin-6 in promoting the growth of human myeloma cells. Oncogene 2002, 21, 2584–2592. [Google Scholar] [CrossRef] [Green Version]
- Moreaux, J.; Cremer, F.W.; Rème, T.; Raab, M.; Mahtouk, K.; Kaukel, P.; Pantesco, V.; De Vos, J.; Jourdan, E.; Jauch, A.; et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 2005, 106, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Tai, Y.-T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel anti–B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Anderson, L.; Sutherland, H.J.; Yong, K.; et al. Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): A dose escalation and expansion phase 1 trial. Lancet Oncol. 2018, 19, 1641–1653. [Google Scholar] [CrossRef]
- Kinneer, K.; Flynn, M.; Thomas, S.B.; Meekin, J.; Varkey, R.; Xiao, X.; Zhong, H.; Breen, S.; Hynes, P.G.; Fleming, R.; et al. Preclinical assessment of an antibody–PBD conjugate that targets BCMA on multiple myeloma and myeloma progenitor cells. Leukemia 2019, 33, 766–771. [Google Scholar] [CrossRef]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Krönke, J.; Facon, T.; Salnikov, A.V.; Lesley, R.; et al. Anti–B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J. Clin. Oncol. 2020, 38, 775–783. [Google Scholar] [CrossRef]
- Hipp, S.; Tai, Y.-T.; Blanset, D.; Deegen, P.; Wahl, J.; Thomas, O.; Rattel, B.; Adam, P.J.; Anderson, K.C.; Friedrich, M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 2017, 31, 1743–1751. [Google Scholar] [CrossRef]
- Ali, S.A.; Shi, V.; Maric, I.; Wang, M.; Stroncek, D.F.; Rose, J.J.; Brudno, J.N.; Stetler-Stevenson, M.; Feldman, S.A.; Hansen, B.G.; et al. T cells expressing an anti–B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016, 128, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Marić, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Stetler-Stevenson, M.; Salem, D.; Yuan, C.; Pavletic, S.; et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Liu, J.; Wang, B.-Y.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Staehr, M.; Masakayan, R.; Tatake, I.J.; Purdon, T.; Wang, X.; Wang, P.; Liu, H.; Xu, Y.; Garrett-Thomson, S.C.; et al. Development and Evaluation of an Optimal Human Single-Chain Variable Fragment-Derived BCMA-Targeted CAR T Cell Vector. Mol. Ther. 2018, 26, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Chen, L.-J.; Yang, S.-S.; Sun, Y.; Wu, W.; Liu, Y.-F.; Zhuang, Y.; Zhang, W.; Weng, X.-Q.; Wu, J.; et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc. Natl. Acad. Sci. USA 2019, 116, 9543–9551. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.A.; Hoffmann, F.S.; Kuhn, P.-H.; Cheng, Q.; Chu, Y.; Schmidt-Supprian, M.; Hauck, S.; Schuh, E.; Krumbholz, M.; Rübsamen, H.; et al. γ-secretase directly sheds the survival receptor BCMA from plasma cells. Nat. Commun. 2015, 6, 7333. [Google Scholar] [CrossRef]
- Sanchez, E.; Smith, E.J.; Yashar, M.A.; Patil, S.; Li, M.; Porter, A.L.; Tanenbaum, E.J.; Schlossberg, R.E.; Soof, C.M.; Hekmati, T.; et al. The Role of B-Cell Maturation Antigen in the Biology and Management of, and as a Potential Therapeutic Target in, Multiple Myeloma. Target. Oncol. 2018, 13, 39–47. [Google Scholar] [CrossRef]
- Pont, M.J.; Hill, T.; Cole, G.O.; Abbott, J.J.; Kelliher, J.; Salter, A.I.; Hudecek, M.; Comstock, M.L.; Rajan, A.; Patel, B.K.R.; et al. Gamma-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood 2019, 134, 1585–1597. [Google Scholar] [CrossRef]
- Kukreja, A.; Hutchinson, A.; Dhodapkar, K.; Mazumder, A.; Vesole, D.; Angitapalli, R.; Jagannath, S.; Dhodapkar, M.V. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J. Exp. Med. 2006, 203, 1859–1865. [Google Scholar] [CrossRef]
- Yaccoby, S.; Pennisi, A.; Li, X.; Dillon, S.R.; Zhan, F.; Barlogie, B.; Shaughnessy, J.D. Atacicept (TACI-Ig) inhibits growth of TACIhigh primary myeloma cells in SCID-hu mice and in coculture with osteoclasts. Leukemia 2008, 22, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Guadagnoli, M.; Kimberley, F.C.; Phan, U.; Cameron, K.; Vink, P.M.; Rodermond, H.; Eldering, E.; Kater, A.P.; Van Eenennaam, H.; Medema, J.P.; et al. Development and characterization of APRIL antagonistic monoclonal antibodies for treatment of B-cell lymphomas. Blood 2011, 117, 6856–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, Y.-T.; Acharya, C.; An, G.; Moschetta, M.; Zhong, M.Y.; Feng, X.; Cea, M.; Cagnetta, A.; Wen, K.; Van Eenennaam, H.; et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016, 127, 3225–3236. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.; Draper, B.; Chaplin, N.; Philip, B.; Chin, M.; Galas-Filipowicz, D.; Onuoha, S.; Thomas, S.; Baldan, V.; Bughda, R.; et al. An APRIL-based chimeric antigen receptor for dual targeting of BCMA and TACI in multiple myeloma. Blood 2018, 131, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, A.; Ormhøj, M.; Choi, B.D.; Taylor, A.O.; Bouffard, A.A.; Scarfò, I.; Larson, R.C.; Frigault, M.J.; Gallagher, K.; Castano, A.P.; et al. Rational design of a trimeric APRIL-based CAR-binding domain enables efficient targeting of multiple myeloma. Blood Adv. 2019, 3, 3248–3260. [Google Scholar] [CrossRef]


| Year | Major Findings | Reference |
|---|---|---|
| 1997 | TACI was cloned as a calcium-modulator and cyclophilin ligand (CAML) associated protein belonging to tumor necrosis factor receptor superfamily. Engagement of TACI led to activation of transcription factors AP-1, NF-AT, and NF-κB. | [34] |
| 2000 | TACI was identified as a receptor that binds BAFF and APRIL. | [15,22,23,24,25,35] |
| 2001 | TACI-deficient mice exhibited increased B cells and splenomegaly, whereas TACI-Ig transgenic mice had defective B cell development and reduced circulating immunoglobulins. | [36,37,38] |
| 2005 | TACI is important for immunoglobulin class switching in murine B cells. Mutations of TACI are associated with common variable immunodeficiency in humans | [39,40,41] |
| 2007 | Engagement of TACI promotes plasmablast differentiation in vitro. TACI-deficient B cells had defective plasma cell differentiation in response to T cell-independent type 2 antigens | [42,43] |
| 2008 | TACI is solely activated by oligomeric BAFF and APRIL and required for the survival of plasmblasts. | [44] |
| 2009 | TACI drives plasma cell differentiation in LPS-activated B cells and is required for T cell-independent type 1 antibody response. | [45] |
| 2010 | TACI signals via associating with adaptor MyD88 to trigger immunoglobulin class switching. | [46] |
| 2011 | TACI is important for differentiation of long-lived antibody-secreting cells by promoting sustained Blimp-1 expression. | [47] |
| 2012 | TACI promotes the survival of long-lived antibody-secreting cells by mediating APRIL-induced downregulation of pro-apoptotic gene Bim. | [48] |
| 2015 | Overexpression of short isoform of TACI in human pre-B cells promotes their differentiation into plasma cells. | [49] |
| 2017 | BAFF-mediated IL-10 production by Breg cells via TACI in healthy donors and CLL patients | [50] |
| 2019 | APRIL increases number of IL-10-producing Breg cells and their IL-10 production via TACI expressed on Breg cells from the bone marrow of MM patients. | [51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Lam, K.-P. Transmembrane Activator and CAML Interactor (TACI): Another Potential Target for Immunotherapy of Multiple Myeloma? Cancers 2020, 12, 1045. https://doi.org/10.3390/cancers12041045
Xu S, Lam K-P. Transmembrane Activator and CAML Interactor (TACI): Another Potential Target for Immunotherapy of Multiple Myeloma? Cancers. 2020; 12(4):1045. https://doi.org/10.3390/cancers12041045
Chicago/Turabian StyleXu, Shengli, and Kong-Peng Lam. 2020. "Transmembrane Activator and CAML Interactor (TACI): Another Potential Target for Immunotherapy of Multiple Myeloma?" Cancers 12, no. 4: 1045. https://doi.org/10.3390/cancers12041045