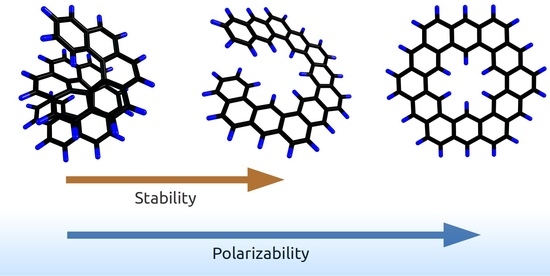
Polarizability of Kekulene, Septulene, and Nearest Non-Planar Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Computation Details
3. Results and Discussion
3.1. Objects under Study
3.2. Thermodynamic Parameters of the Studied PAHs
3.3. Mean Polarizabilities of the Studied PAHs
3.4. General Remarks and Prospective
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clar, E. Polycyclic Hydrocarbons; Academic Press: New York, NY, USA, 1964. [Google Scholar] [CrossRef]
- Slayden, S.W.; Liebman, J.F. The energetics of aromatic hydrocarbons: An experimental thermochemical perspective. Chem. Rev. 2001, 101, 1541–1566. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, C.-F. Helicenes: Synthesis and Applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef]
- Kiel, G.R.; Patel, S.C.; Smith, P.W.; Levine, D.S.; Tilley, T.D. Expanded Helicenes: A General Synthetic Strategy and Remarkable Supramolecular and Solid-State Behavior. J. Am. Chem. Soc. 2017, 139, 18456–18459. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Hernández-Trujillo, J.; Tang, T.-H.; Bader, R.F.W. Hydrogen–hydrogen bonding: A stabilizing interaction in molecules and crystals. Chem. Eur. J. 2003, 9, 1940–1951. [Google Scholar] [CrossRef]
- Vashchenko, A.V.; Borodina, T.N. H–H interaction in phenanthrene: Attraction or repulsion? J. Struct. Chem. 2013, 54, 479–483. [Google Scholar] [CrossRef]
- Poater, J.; Visser, R.; Solà, M.; Bickelhaupt, F.M. Polycyclic benzenoids: Why kinked is more stable than straight. J. Org. Chem. 2007, 72, 1134–1142. [Google Scholar] [CrossRef]
- Portella, G.; Poater, J.; Bofill, J.M.; Alemany, P.; Solà, M. Local aromaticity of [n]acenes, [n]phenacenes, and [n]helicenes (n = 1–9). J. Org. Chem. 2005, 70, 2509–2521. [Google Scholar] [CrossRef]
- Sabirov, D. A correlation between the mean polarizability of the “kinked” polycyclic aromatic hydrocarbons and the number of H...H bond critical points predicted by Atoms-in-Molecules theory. Comput. Theor. Chem. 2014, 1030, 81–86. [Google Scholar] [CrossRef]
- Sabirov, D.; Garipova, R.R.; Cataldo, F. Polarizability of isomeric and related interstellar compounds in the aspect of their abundance. Mol. Astrophys. 2018, 12, 10–19. [Google Scholar] [CrossRef]
- Grandberg, I.I. Organic Chemistry; Mir: Moscow, Russia, 2002. [Google Scholar]
- Ehrenfreund, P.; Ruiterkamp, R.; Peeters, Z.; Foing, B.; Salama, F.; Martins, Z. The ORGANICS experiment on BIOPAN V: UV and space exposure of aromatic compounds. Planet. Space Sci. 2007, 55, 383–400. [Google Scholar] [CrossRef]
- Kwok, S.; Zhang, Y. Mixed aromatic–aliphatic organic nanoparticles as carriers of unidentified infrared emission features. Nature 2011, 479, 80–83. [Google Scholar] [CrossRef]
- Zhao, L.; Kaiser, R.I.; Xu, B.; Ablikim, U.; Lu, W.; Ahmed, M.; Evseev, M.M.; Bashkirov, E.K.; Azyazov, V.N.; Zagidullin, M.V.; et al. Gas phase synthesis of [4]-helicene. Nat. Commun. 2019, 10, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oña-Ruales, J.O.; Ruiz-Morales, Y.; Alvarez-Ramírez, F. The Helicenes: Potential carriers of diffuse interstellar bands. ACS Earth Space Chem. 2021, 5, 381–390. [Google Scholar] [CrossRef]
- Sadjadi, S.; Kwok, S.; Zhang, Y. Theoretical infrared spectra of MAON molecules. J. Phys. Conf. Ser. 2016, 728, 62003. [Google Scholar] [CrossRef] [Green Version]
- Sabirov, D.S.; Tukhbatullina, A.A.; Shepelevich, I.S. Polarizability in astrochemical studies of complex carbon-based compounds. ACS Earth Space Chem. 2022, 6, 1–17. [Google Scholar] [CrossRef]
- Alparone, A.; Librando, V.; Minniti, Z. Validation of semiempirical PM6 method for the prediction of molecular properties of polycyclic aromatic hydrocarbons and fullerenes. Chem. Phys. Lett. 2008, 460, 151–154. [Google Scholar] [CrossRef]
- Martin, D.; Sild, S.; Maran, U.; Karelson, M. QSPR modeling of the polarizability of polyaromatic hydrocarbons and fullerenes. J. Phys. Chem. C 2008, 112, 4785–4790. [Google Scholar] [CrossRef]
- Smith, S.M.; Markevitch, A.N.; Romanov, D.A.; Li, X.; Levis, R.J.; Schlegel, H.B. Static and dynamic polarizabilities of conjugated molecules and their cations. J. Phys. Chem. A 2004, 108, 11063–11072. [Google Scholar] [CrossRef] [Green Version]
- Sabirov, D.S.; Terentyev, A.O.; Bulgakov, R.G. Polarizability of fullerene [2+2]-dimers: A DFT study. Phys. Chem. Chem. Phys. 2014, 16, 14594–14600. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Sabirov, D.S. Polarizability of C60 fullerene dimer and oligomers: The unexpected enhancement and its use for rational design of fullerene-based nanostructures with adjustable properties. RSC Adv. 2013, 3, 19430–19439. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Terentyev, A.O.; Cataldo, F. Bisadducts of the C60 and C70 fullerenes with anthracene: Isomerism and DFT estimation of stability and polarizability. Comput. Theor. Chem. 2016, 1081, 44–48. [Google Scholar] [CrossRef]
- Nénon, S.; Champagne, B.R.; Spassova, M. Assessing long-range corrected functionals with physically-adjusted range-separated parameters for calculating the polarizability and the second hyperpolarizability of polydiacetylene and polybutatriene chains. Phys. Chem. Chem. Phys. 2014, 16, 7083–7088. [Google Scholar] [CrossRef] [PubMed]
- Le Fèvre, R.J.W.; Sundaram, K.M.S. Molecular polarisability. The molar Kerr constants, polarisations, etc., of ten polynuclear hydrocarbons as solutes in benzene. J. Chem. Soc. 1963, 4442–4446. [Google Scholar] [CrossRef]
- Diederich, F.; Staab, H.A. Benzenoid versus annulenoid aromaticity: Synthesis and properties of kekulene. Angew. Chem. Int. Ed. Engl. 1978, 17, 372–374. [Google Scholar] [CrossRef]
- Poater, J.; Paauwe, J.; Pan, S.; Merino, G.; Fonseca-Guerra, C.; Bickelhaupt, F.M. Kekulene: Structure, stability and nature of H…H interactions in large PAHs. Mol. Astrophys. 2017, 8, 19–26. [Google Scholar] [CrossRef]
- Haags, A.; Reichmann, A.; Fan, Q.; Egger, L.; Kirschner, H.; Naumann, T.; Werner, S.; Vollgraff, T.; Sundermeyer, J.; Eschmann, L.; et al. Kekulene: On-surface synthesis, orbital structure, and aromatic stabilization. ACS Nano 2020, 14, 15766–15775. [Google Scholar] [CrossRef]
- Kumar, B.; Viboh, R.L.; Bonifacio, M.C.; Thompson, W.B.; Buttrick, J.C.; Westlake, B.C.; Kim, M.-S.; Zoellner, R.W.; Varganov, S.A.; Mörschel, P.; et al. Septulene: The Heptagonal Homologue of Kekulene. Angew. Chem. Int. Ed. 2012, 51, 12795–12800. [Google Scholar] [CrossRef]
- Ghanty, T.K.; Ghosh, S.K. A density functional approach to hardness, polarizability, and valency of molecules in chemical reactions. J. Phys. Chem. 1996, 100, 12295–12298. [Google Scholar] [CrossRef]
- Sabirov, D.; Garipova, R.R.; Kinzyabaeva, Z.S. Fullerene–1,4-dioxane adducts: A DFT study of the structural features and molecular properties. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 154–159. [Google Scholar] [CrossRef]
- Sharipov, A.S.; Loukhovistky, B.I. Small atomic clusters: Quantum chemical research of isomeric composition and physical properties. Struct. Chem. 2019, 30, 2057–2084. [Google Scholar] [CrossRef]
- Blair, S.A.; Thakkar, A.J. How often is the minimum polarizability principle violated? Chem. Phys. Lett. 2013, 556, 346–349. [Google Scholar] [CrossRef]
- Hohm, U. Is there a minimum polarizability principle in chemical reactions? J. Phys. Chem. A 2000, 104, 8418–8423. [Google Scholar] [CrossRef]
- Vereshchagin, A.N. Polarizability of Molecules; Nauka: Moscow, Russia, 1980. [Google Scholar]
- Dolomatov, M.Y.; Burangulov, D.Z.; Dolomatova, M.M.; Osipenko, D.F.; Zaporin, V.P.; Tukhbatullina, A.A.; Akhmetov, A.F.; Sabirov, D.S. Low-sulphur vacuum gasoil of Western Siberia oil: The impact of its structural and chemical features on the properties of the produced needle coke. C 2022, 8, 19. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kaji, Y.; Ogawa, K.; Eguchi, R.; Oikawa, S.; Gohda, H.; Fujiwara, A.; Kubozono, Y. Characteristics of field-effect transistors using the one-dimensional extended hydrocarbon [7]phenacene. Appl. Phys. Lett. 2011, 98, 013303. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Ori, O.; Tukhbatullina, A.A.; Shepelevich, I.S. Structural descriptors of benzenoid hydrocarbons: A mismatch between the estimates and parity effects in helicenes. C 2022, 8, 42. [Google Scholar] [CrossRef]
- Lukmanov, T.I.; Shepelevich, I.S.; Sabirov, D.S. Polarizability of polycyclic aromatic hydrocarbon compounds from the intermediate stages of the oxidative condensation of hexaphenylbenzene into hexa-peri-benzocoronene. Vestn. Bashkir. Univ. 2022, 27, 98–101. [Google Scholar] [CrossRef]


| Molecule | α (Å3) | ΔαR (Å3) a | (kJ/mol) b |
|---|---|---|---|
| [11]helicene | 80.1 | n/a | n/a |
| [11]helicene-exp | 102.9 | n/a | n/a |
| kekulene | 111.1 | n/a | n/a |
| [12]helicene | 86.3 | −28.6 | 126.9 |
| [12]helicene-exp | 112.6 | −2.3 | −43.0 |
| [13]circulene | 123.0 | n/a | n/a |
| [13]helicene | 92.3 | −34.5 | 90.5 |
| [13]helicene-exp | 119.1 | −7.7 | −107.2 |
| septulene | 135.5 | n/a | n/a |
| [14]helicene | 98.5 | −40.8 | 141.4 |
| [14]helicene-exp | 127.8 | −11.4 | −72.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukmanov, T.; Akhmetov, A.F.; Sabirov, D.S. Polarizability of Kekulene, Septulene, and Nearest Non-Planar Polycyclic Aromatic Hydrocarbons. C 2022, 8, 61. https://doi.org/10.3390/c8040061
Lukmanov T, Akhmetov AF, Sabirov DS. Polarizability of Kekulene, Septulene, and Nearest Non-Planar Polycyclic Aromatic Hydrocarbons. C. 2022; 8(4):61. https://doi.org/10.3390/c8040061
Chicago/Turabian StyleLukmanov, Timur, Arslan F. Akhmetov, and Denis Sh. Sabirov. 2022. "Polarizability of Kekulene, Septulene, and Nearest Non-Planar Polycyclic Aromatic Hydrocarbons" C 8, no. 4: 61. https://doi.org/10.3390/c8040061