Effects of Paired Associative Stimulation on Cortical Plasticity in Agonist–Antagonist Muscle Representations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
2.3. Electromyographic Recordings
2.4. Transcranial Magnetic Stimulation
2.5. Motor Representational Map
2.6. Paired Associative Stimulation
2.7. Cortical Excitability Recordings
2.8. Data Analysis
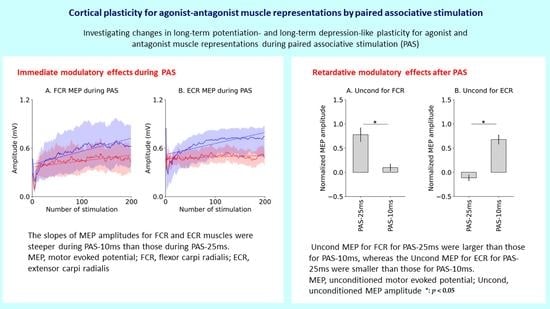
2.8.1. Immediate Modulatory Effects during PAS
2.8.2. Retardative Modulatory Effects after PAS
2.9. Statistical Analysis
3. Results
3.1. Motor Representational Map
3.2. Immediate Modulatory Effects during PAS
3.3. Retardative Modulatory Effects after PAS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kariminezhad, S.; Karhu, J.; Saisanen, L.; Reijonen, J.; Kononen, M.; Julkunen, P. Brain Response Induced with Paired Associative Stimulation Is Related to Repetition Suppression of Motor Evoked Potential. Brain Sci. 2020, 10, 674. [Google Scholar] [CrossRef] [PubMed]
- Stefan, K.; Kunesch, E.; Cohen, L.G.; Benecke, R.; Classen, J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000, 123 Pt 3, 572–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, D.E. Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex. Neuron 2000, 27, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, S.F.; Bliss, T.V. Plasticity in the human central nervous system. Brain 2006, 129, 1659–1673. [Google Scholar] [CrossRef] [Green Version]
- Bi, G.Q.; Poo, M.M. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 1998, 18, 10464–10472. [Google Scholar] [CrossRef] [Green Version]
- Ziemann, U.; Ilic, T.V.; Pauli, C.; Meintzschel, F.; Ruge, D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J. Neurosci. 2004, 24, 1666–1672. [Google Scholar] [CrossRef] [Green Version]
- Muller-Dahlhaus, F.; Ziemann, U.; Classen, J. Plasticity resembling spike-timing dependent synaptic plasticity: The evidence in human cortex. Front. Synaptic Neurosci. 2010, 2, 34. [Google Scholar] [CrossRef] [Green Version]
- Wolters, A.; Sandbrink, F.; Schlottmann, A.; Kunesch, E.; Stefan, K.; Cohen, L.G.; Benecke, R.; Classen, J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J. Neurophysiol. 2003, 89, 2339–2345. [Google Scholar] [CrossRef]
- Stefan, K.; Kunesch, E.; Benecke, R.; Cohen, L.G.; Classen, J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J. Physiol. 2002, 543, 699–708. [Google Scholar] [CrossRef]
- Castel-Lacanal, E.; Gerdelat-Mas, A.; Marque, P.; Loubinoux, I.; Simonetta-Moreau, M. Induction of cortical plastic changes in wrist muscles by paired associative stimulation in healthy subjects and post-stroke patients. Exp. Brain Res. 2007, 180, 113–122. [Google Scholar] [CrossRef]
- Caria, M.A.; Kaneko, T.; Kimura, A.; Asanuma, H. Functional organization of the projection from area 2 to area 4gamma in the cat. J. Neurophysiol. 1997, 77, 3107–3114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quartarone, A.; Bagnato, S.; Rizzo, V.; Siebner, H.R.; Dattola, V.; Scalfari, A.; Morgante, F.; Battaglia, F.; Romano, M.; Girlanda, P. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain 2003, 126, 2586–2596. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.; Lavender, A.P.; Ridding, M.C.; Semmler, J.G. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J. Physiol. 2009, 587, 5831–5842. [Google Scholar] [CrossRef] [PubMed]
- Russmann, H.; Lamy, J.C.; Shamim, E.A.; Meunier, S.; Hallett, M. Associative plasticity in intracortical inhibitory circuits in human motor cortex. Clin. Neurophysiol. 2009, 120, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Elahi, B.; Gunraj, C.; Chen, R. Short-interval intracortical inhibition blocks long-term potentiation induced by paired associative stimulation. J. Neurophysiol. 2012, 107, 1935–1941. [Google Scholar] [CrossRef] [Green Version]
- Carson, R.G.; Kennedy, N.C. Modulation of human corticospinal excitability by paired associative stimulation. Front. Hum. Neurosci. 2013, 7, 823. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Kirimoto, H.; Sugawara, K.; Watanabe, M.; Shimizu, S.; Ishizaka, I.; Yamada, S.; Matsunaga, A.; Fukuda, M.; Onishi, H. Induction of cortical plasticity for reciprocal muscles by paired associative stimulation. Brain Behav. 2014, 4, 822–832. [Google Scholar] [CrossRef]
- Tarri, M.; Brihmat, N.; Gasq, D.; Lepage, B.; Loubinoux, I.; De Boissezon, X.; Marque, P.; Castel-Lacanal, E. Five-day course of paired associative stimulation fails to improve motor function in stroke patients. Ann. Phys. Rehabil. Med. 2018, 61, 78–84. [Google Scholar] [CrossRef]
- Morgante, F.; Espay, A.J.; Gunraj, C.; Lang, A.E.; Chen, R. Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain 2006, 129, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Ueki, Y.; Mima, T.; Kotb, M.A.; Sawada, H.; Saiki, H.; Ikeda, A.; Begum, T.; Reza, F.; Nagamine, T.; Fukuyama, H. Altered plasticity of the human motor cortex in Parkinson’s disease. Ann. Neurol. 2006, 59, 60–71. [Google Scholar] [CrossRef]
- Orth, M.; Schippling, S.; Schneider, S.A.; Bhatia, K.P.; Talelli, P.; Tabrizi, S.J.; Rothwell, J.C. Abnormal motor cortex plasticity in premanifest and very early manifest Huntington disease. J. Neurol. Neurosurg. Psychiatry 2010, 81, 267–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huntley, G.W.; Jones, E.G. Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: A correlative anatomic and physiological study. J. Neurophysiol. 1991, 66, 390–413. [Google Scholar] [CrossRef] [PubMed]
- Melgari, J.M.; Pasqualetti, P.; Pauri, F.; Rossini, P.M. Muscles in “concert”: Study of primary motor cortex upper limb functional topography. PLoS ONE 2008, 3, e3069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Kirimoto, H.; Onishi, H.; Yamada, S.; Tamaki, H.; Maruyama, A.; Yamamoto, J. Reciprocal changes in input-output curves of motor evoked potentials while learning motor skills. Brain Res. 2012, 1473, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kirimoto, H.; Sugawara, K.; Oyama, M.; Yamada, S.; Yamamoto, J.; Matsunaga, A.; Fukuda, M.; Onishi, H. Motor cortex-evoked activity in reciprocal muscles is modulated by reward probability. PLoS ONE 2014, 9, e90773. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hamaguchi, T.; Matsunaga, A. Nonequivalent modulation of corticospinal excitability by positive and negative outcomes. Brain Behav. 2018, 8, e00862. [Google Scholar] [CrossRef] [Green Version]
- Kwan, H.C.; MacKay, W.A.; Murphy, J.T.; Wong, Y.C. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J. Neurophysiol. 1978, 41, 1120–1131. [Google Scholar] [CrossRef]
- Sessle, B.J.; Wiesendanger, M. Structural and functional definition of the motor cortex in the monkey (Macaca fascicularis). J. Physiol. 1982, 323, 245–265. [Google Scholar] [CrossRef]
- Gerachshenko, T.; Stinear, J.W. Suppression of motor evoked potentials in biceps brachii preceding pronator contraction. Exp. Brain Res. 2007, 183, 531–539. [Google Scholar] [CrossRef]
- Giacobbe, V.; Volpe, B.T.; Thickbroom, G.W.; Fregni, F.; Pascual-Leone, A.; Krebs, H.I.; Edwards, D.J. Reversal of TMS-induced motor twitch by training is associated with a reduction in excitability of the antagonist muscle. J. Neuroeng. Rehabil. 2011, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Leone, A.; Nguyet, D.; Cohen, L.G.; Brasil-Neto, J.P.; Cammarota, A.; Hallett, M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 1995, 74, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J.; Milliken, G.W.; Jenkins, W.M.; Merzenich, M.M. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J. Neurosci. 1996, 16, 785–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanes, J.N.; Donoghue, J.P. Plasticity and primary motor cortex. Annu. Rev. Neurosci. 2000, 23, 393–415. [Google Scholar] [CrossRef] [Green Version]
- Kleim, J.A.; Kleim, E.D.; Cramer, S.C. Systematic assessment of training-induced changes in corticospinal output to hand using frameless stereotaxic transcranial magnetic stimulation. Nat. Protoc. 2007, 2, 1675–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaka, Y.; Picard, N.; Strick, P.L. Skill representation in the primary motor cortex after long-term practice. J. Neurophysiol. 2007, 97, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Siebner, H.R.; Rothwell, J. Transcranial magnetic stimulation: New insights into representational cortical plasticity. Exp. Brain Res. 2003, 148, 1–16. [Google Scholar] [CrossRef]
- Meesen, R.L.; Cuypers, K.; Rothwell, J.C.; Swinnen, S.P.; Levin, O. The effect of long-term TENS on persistent neuroplastic changes in the human cerebral cortex. Hum. Brain Mapp. 2011, 32, 872–882. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Bikson, M.; Hanlon, C.A.; Woods, A.J.; Gillick, B.T.; Charvet, L.; Lamm, C.; Madeo, G.; Holczer, A.; Almeida, J.; Antal, A.; et al. Guidelines for TMS/tES clinical services and research through the COVID-19 pandemic. Brain Stimul. 2020, 13, 1124–1149. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Valenzano, A.; Moscatelli, F.; Salerno, M.; Sessa, F.; Triggiani, A.I.; Viggiano, A.; Capranica, L.; Marsala, G.; De Luca, V.; et al. Primary Motor Cortex Excitability in Karate Athletes: A Transcranial Magnetic Stimulation Study. Front. Physiol. 2017, 8, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julkunen, P.; Ruohonen, J.; Saaskilahti, S.; Saisanen, L.; Karhu, J. Threshold curves for transcranial magnetic stimulation to improve reliability of motor pathway status assessment. Clin. Neurophysiol. 2011, 122, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Marconi, B.; Filippi, G.M.; Koch, G.; Giacobbe, V.; Pecchioli, C.; Versace, V.; Camerota, F.; Saraceni, V.M.; Caltagirone, C. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil. Neural Repair 2011, 25, 48–60. [Google Scholar] [CrossRef]
- Delvendahl, I.; Jung, N.H.; Mainberger, F.; Kuhnke, N.G.; Cronjaeger, M.; Mall, V. Occlusion of bidirectional plasticity by preceding low-frequency stimulation in the human motor cortex. Clin. Neurophysiol. 2010, 121, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Terranova, C.; Hilker, R.; Quartarone, A.; Ziemann, U. Deficient homeostatic regulation of practice-dependent plasticity in writer’s cramp. Cereb. Cortex 2011, 21, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Voytovych, H.; Krivanekova, L.; Ziemann, U. Lithium: A switch from LTD- to LTP-like plasticity in human cortex. Neuropharmacology 2012, 63, 274–279. [Google Scholar] [CrossRef]
- He, X.K.; Sun, Q.Q.; Liu, H.H.; Guo, X.Y.; Chen, C.; Chen, L.D. Timing of Acupuncture during LTP-Like Plasticity Induced by Paired-Associative Stimulation. Behav. Neurol. 2019, 2019, 9278270. [Google Scholar] [CrossRef]
- Weise, D.; Schramm, A.; Stefan, K.; Wolters, A.; Reiners, K.; Naumann, M.; Classen, J. The two sides of associative plasticity in writer’s cramp. Brain 2006, 129, 2709–2721. [Google Scholar] [CrossRef]
- Weise, D.; Schramm, A.; Beck, M.; Reiners, K.; Classen, J. Loss of topographic specificity of LTD-like plasticity is a trait marker in focal dystonia. Neurobiol. Dis. 2011, 42, 171–176. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.F.; Thirugnanasambandam, N.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J. Neurosci. 2009, 29, 6124–6131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thirugnanasambandam, N.; Grundey, J.; Adam, K.; Drees, A.; Skwirba, A.C.; Lang, N.; Paulus, W.; Nitsche, M.A. Nicotinergic impact on focal and non-focal neuroplasticity induced by non-invasive brain stimulation in non-smoking humans. Neuropsychopharmacology 2011, 36, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amadi, U.; Allman, C.; Johansen-Berg, H.; Stagg, C.J. The Homeostatic Interaction Between Anodal Transcranial Direct Current Stimulation and Motor Learning in Humans is Related to GABAA Activity. Brain Stimul. 2015, 8, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Guerra, A.; Pogosyan, A.; Nowak, M.; Tan, H.; Ferreri, F.; Di Lazzaro, V.; Brown, P. Phase Dependency of the Human Primary Motor Cortex and Cholinergic Inhibition Cancelation During Beta tACS. Cereb. Cortex 2016, 26, 3977–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, M.; Tanaka, S.; Gomez-Tames, J.; Okabe, T.; Cho, K.; Iso, N.; Hirata, A. Nonequivalent After-Effects of Alternating Current Stimulation on Motor Cortex Oscillation and Inhibition: Simulation and Experimental Study. Brain Sci. 2022, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Snijders, A.H.; Rothwell, J.C. The variability of intracortical inhibition and facilitation. Clin. Neurophysiol. 2003, 114, 2362–2369. [Google Scholar] [CrossRef]
- Ziemann, U.; Rothwell, J.C.; Ridding, M.C. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. 1996, 496 Pt 3, 873–881. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- van der Spoel, E.; Choi, J.; Roelfsema, F.; Cessie, S.L.; van Heemst, D.; Dekkers, O.M. Comparing Methods for Measurement Error Detection in Serial 24-h Hormonal Data. J. Biol. Rhythm. 2019, 34, 347–363. [Google Scholar] [CrossRef]
- Kiers, L.; Cros, D.; Chiappa, K.H.; Fang, J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 415–423. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, T.; Wang, Y.J.; Hamaguchi, T. Changes in Magnitude and Variability of Corticospinal Excitability During Rewarded Time-Sensitive Behavior. Front. Behav. Neurosci. 2019, 13, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajji, T.K.; Liu, S.K.; Frantseva, M.V.; Mulsant, B.H.; Thoma, J.; Chen, R.; Fitzgerald, P.B.; Daskalakis, Z.J. Exploring the effect of inducing long-term potentiation in the human motor cortex on motor learning. Brain. Stimul. 2011, 4, 137–144. [Google Scholar] [CrossRef] [PubMed]
- McGie, S.C.; Masani, K.; Popovic, M.R. Failure of spinal paired associative stimulation to induce neuroplasticity in the human corticospinal tract. J. Spinal Cord Med. 2014, 37, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, R.; Maatta, S.; Esser, S.K.; Sarasso, S.; Ferrarelli, F.; Watson, A.; Ferreri, F.; Peterson, M.J.; Tononi, G. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J. Neurosci. 2008, 28, 7911–7918. [Google Scholar] [CrossRef] [Green Version]
- Bienenstock, E.L.; Cooper, L.N.; Munro, P.W. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J. Neurosci. 1982, 2, 32–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, W.C. Metaplasticity: Tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 2008, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Elahi, B.; Hutchison, W.D.; Daskalakis, Z.J.; Gunraj, C.; Chen, R. Dose-response curve of associative plasticity in human motor cortex and interactions with motor practice. J. Neurophysiol. 2014, 111, 594–601. [Google Scholar] [CrossRef] [Green Version]
- De Beaumont, L.; Tremblay, S.; Poirier, J.; Lassonde, M.; Theoret, H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb. Cortex 2012, 22, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Paille, V.; Fino, E.; Du, K.; Morera-Herreras, T.; Perez, S.; Kotaleski, J.H.; Venance, L. GABAergic circuits control spike-timing-dependent plasticity. J. Neurosci. 2013, 33, 9353–9363. [Google Scholar] [CrossRef] [Green Version]
- Pitkanen, M.; Yazawa, S.; Airaksinen, K.; Lioumis, P.; Nurminen, J.; Pekkonen, E.; Makela, J.P. Localization of Sensorimotor Cortex Using Navigated Transcranial Magnetic Stimulation and Magnetoencephalography. Brain Topogr. 2019, 32, 873–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Center of Amplitude (mm) | ||||
|---|---|---|---|---|
| FCR | ECR | |||
| Anteroposterior | Mediolateral | Anteroposterior | Mediolateral | |
| Before PAS-25 ms Before PAS-10 ms After PAS-25 ms After PAS-10 ms | −56.2 ± 1.6 −55.6 ± 1.2 −57.8 ± 1.4 −57.1 ± 1.2 | 4.9 ± 1.7 5.1 ± 2.3 4.3 ± 1.8 5.9 ± 2.2 | −55.7 ± 1.6 −55.0 ± 1.1 −56.9 ± 1.6 −57.2 ± 1.0 | 3.1 ± 1.8 4.3 ± 2.2 3.5 ± 1.9 5.4 ± 2.0 |
| Condition | Muscle | R2 | p | ||
|---|---|---|---|---|---|
| PAS-25 ms | FCR ECR | 0.367 ± 0.138 0.472 ± 0.088 | 0.001 ± 0.000 0.000 ± 0.000 | 0.411 0.150 | <0.0001 <0.0001 |
| PAS-10 ms | FCR ECR | 0.399 ± 0.197 0.520 ± 0.111 | 0.002 ± 0.001 0.001 ± 0.000 | 0.722 0.644 | <0.0001 <0.0001 |
| Before PAS-25 ms | After PAS-25 ms | Before PAS-10 ms | After PAS-10 ms | |||||
|---|---|---|---|---|---|---|---|---|
| FCR | ECR | FCR | ECR | FCR | ECR | FCR | ECR | |
| RMT (%) | 58.3 ± 2.5 | 56.6 ± 1.9 | 57.0 ± 1.9 | 54.6 ± 1.6 | 58.7 ± 2.3 | 55.3 ± 1.8 | 59.6 ± 2.1 | 56.1 ± 1.7 |
| Uncond (mV) | 0.33 ± 0.03 | 0.53 ± 0.04 | 0.31 ± 0.03 | 0.54 ± 0.04 | 0.34 ± 0.02 | 0.43 ± 0.03 | 0.34 ± 0.02 | 0.47 ± 0.03 |
| SICI (mV) | 0.13 ± 0.02 | 0.22 ± 0.02 | 0.14 ± 0.02 | 0.29 ± 0.02 | 0.15 ± 0.02 | 0.26 ± 0.02 | 0.20 ± 0.02 | 0.31 ± 0.03 |
| LICI (mV) | 0.04 ± 0.00 | 0.15 ± 0.02 | 0.03 ± 0.00 | 0.17 ± 0.02 | 0.08 ± 0.01 | 0.14 ± 0.02 | 0.08 ± 0.01 | 0.14 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, M.; Saito, K.; Maeda, Y.; Cho, K.; Iso, N.; Okabe, T.; Suzuki, T.; Yamamoto, J. Effects of Paired Associative Stimulation on Cortical Plasticity in Agonist–Antagonist Muscle Representations. Brain Sci. 2023, 13, 475. https://doi.org/10.3390/brainsci13030475
Suzuki M, Saito K, Maeda Y, Cho K, Iso N, Okabe T, Suzuki T, Yamamoto J. Effects of Paired Associative Stimulation on Cortical Plasticity in Agonist–Antagonist Muscle Representations. Brain Sciences. 2023; 13(3):475. https://doi.org/10.3390/brainsci13030475
Chicago/Turabian StyleSuzuki, Makoto, Kazuo Saito, Yusuke Maeda, Kilchoon Cho, Naoki Iso, Takuhiro Okabe, Takako Suzuki, and Junichi Yamamoto. 2023. "Effects of Paired Associative Stimulation on Cortical Plasticity in Agonist–Antagonist Muscle Representations" Brain Sciences 13, no. 3: 475. https://doi.org/10.3390/brainsci13030475