For the purpose of this study, the electrochemical parameters of titanium dioxide nanotubes with a diameter of 50 ± 5 nm and height of 1000 ± 100 nm, annealed at 450 °C in argon atmosphere for 2 h before and after deposition of gold nanoparticles using cyclic voltammetry (potential from −1.25 V to −0.7 V) [
20] for different number of cycles (8, 20, 40, 60, 80) in 0.1 mM HAuCl
4 were examined. Further analysis compared the titanium dioxide nanotube electrochemical properties in terms of selected number of cycles (8, 20, 40) and different concentration of HAuCl
4 (0.1 mM, 1 mM, 5 mM, 10 mM). The analysis included comparison of the gold nanoparticles with similar diameter but deposited at different number of cycles and different concentrations of tetrachloroauric acid solution. To confirm the possibility of using this platform as a biosensor, BSA protein detection was carried out.
3.1. Characterization of TNTs before and after AuNPs Deposition–Influence of the Number of Cycles
Figure 1a shows SEM micrographs of the surface and cross-section of TNT arrays prepared by anodic oxidation at 17 V in NH
4F/ethylene glycol/H
2O electrolyte solutions, annealed in argon according toa process described in
Section 2.1. The TNT arrays with diameter of 50±5 nm and height of 1000 ± 100 nm had smooth walls without any perforation and were uniformly arranged on the titanium foil. No damage on the TNT layers after annealing at 450°C for 2 h was observed. Thermal modification at 450°C in argon atmosphere for 2 h TNTs enables changing of TiO
2 from amorphous form (originally present in nanotubes) into crystalline form of rutile and/or anatase [
23,
24]. The most important advantage of annealing is formation of oxygen vacancies, resulting in the improvement of nanotubes conductivity and thus, facilitating the transfer of electrons attributed to the conversion of Ti
4+ to Ti
3+ [
25,
26]. It was suggested that thermal modification of Ti/TNTs carried out at 450 °C results in the predominance of anatase in their structure [
2], which has a higher affinity to biomolecules.
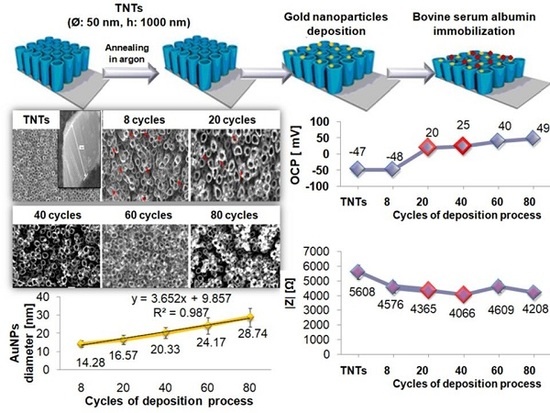
Figure 1b–f shows the SEM images of morphology of Ti/TNTs after deposition of gold nanoparticles. The samples were denoted correspondingly as xAuNPs/TNTs, where x is number of cycles of the deposition process, and x = 8, 20, 40, 60, 80. An [AuCl
4]
— electrolyte solution can be ionized as seen in Equation (3). Au
3+ near the titanium dioxide nanotube arrays can receive electrons and be reduced to Au, according to the Equation (4) [
19]:
Mass transfer in solution occurs by diffusion, migration, and convection, whereas the diffusion and migration result from gradient and electrochemical potential difference respectively, and convection results from an imbalance of forces on the solution. Decrease of Au
3+ concentrations around the TNTs causes the occurrence of the concentration gradient between the bulk solution and the TNTs. Therefore, Au
3+ ions move towards the polarized TNTs surface. As a consequence, more reduced Au crystals are formed on the surface of TNT arrays [
19].
The AuNPs are homogeneously distributed on the surface of the TNT arrays. Due to higher current densities, nucleation mostly takes place at the boundaries between titanium dioxide nanotubes (
Figure 1b–f)—places of the nanotubes contact [
19,
27]. Countless boundaries (
Figure 1a) provided many points of Au nanoparticles nucleation. The homogeneity of TNT arrays provides a homogeneous environment for nucleation of Au nanoparticles [
27]. As can be seen in the
Figure 1e,f, the possibility of nanoparticles aggregation increases with the increase of the number of cycles.
To enhance the electrochemical responses, the size of nanoparticles should be small and homogeneously dispersed on TNT arrays.
Table 1 shows Au components on the surface through EDS analysis in three different places (mean value with SD). Due to low content of gold nanoparticles on the Ti/TNTs surface for the deposition process carried out for 8 and 20 cycles the resulting value of EDS were affected by high measurement error; therefore, it has not been included in
Table 1. The result revealed that the loading amount of Au gradually increases with the increase of the number of deposition cycles from 1.42 ± 0.21 wt% for 40 cycles to 3.59 ± 0.18 wt% for 80 cycles. This result is consistent with the results described by Lianghsen et al. [
20]. Additionally, the increase in the number the deposition cycles causes the increase of the diameter of deposited gold nanoparticles from 14.3 ± 1.8 nm for 8 cycles to 28.7 ± 5.2 nm for 80 cycles, similarly to Babu et al. [
21]. A linear growth (
R2 = 0.998) between the number of CV cycles and diameter of gold nanoparticles deposited on the TNT arrays (
Figure 2) may be observed. However, increase of the number of cycles causes high deviation of gold nanoparticles diameter—a higher value of SD. According to Mahmud et al. [
28], AuNPs deposition on annealed TNTs surface compared to non-annealed TNTs surface promotes agglomeration around the pore of the titanium dioxide nanotubes with a rather poor size distribution.
Table 1 shows the open-circuit potential average values for annealed TNTs samples before and after Au nanoparticles deposition process. After the AuNPs deposition, OCP of the samples is further enhanced compared to non-modified TNT arrays
. Au nanoparticles deposited during eight cycles of cyclic voltammetry, indicating the lowest content of gold (based on EDS analysis), caused no change in the OCP value compared to TNTs. AuNPs deposition carried out for 20–80 cycles causes the general trend for the OCP values to increase. It can be explained by two factors, i.e., the homogeneous AuNPs distribution on the surface of the TNT arrays and the inherent inertness of gold [
19]. For AuNPs/TNTs, deposition in cyclic voltammetry process carried out for 20–80 cycles, a positive charge of the surface was observed. The negatively charged protein molecules are easily attracted to the positively-charged matrix, which might be used in construction of the biosensing platforms.
The Nyquist diagrams (
Figure 3a) determined for the titanium dioxide nanotube layers before and after the deposition of nanoparticles present fragments of wide, incomplete semicircles characteristic of thin oxide layers [
29]. The values recorded in the lowest frequency (0.1 Hz) presented in
Table A1 and
Figure 3, show that the electrochemical parameters depend on the diameter of Au nanoparticles. Due to good electrical conductivity of the AuNPs, the impedance modulus of TNTs decreases. For 60 and 80 cycles, the impedance modulus value slightly increases. This may result from the formation of agglomerates on the TNTs surface (nanoparticles with a diameter in the range from 24.2 ± 4.4 nm to 28.7 ± 5.2 nm). As presented in
Figure 1e,f, many of the nanoparticles are deposited on the inner surface of the titanium dioxide nanotubes, which causes their partial blockage. In addition, the ratio of the gold nanoparticle surface area to their volume is reduced, thus their electrochemical responses deteriorate. It can be noticed that the real impedance (ReZ) value of the xAuNPs/TNTs decreases when compared to non-modified TNT layers. However, this value increases with the increase of the gold nanoparticle diameters. The lowest impedance value 4066 ± 94 Ω and imaginary impedance 4051 ± 97 Ω was noted for nanoparticles with a diameter of 20.3 ± 2.9 nm (40AuNPs/TNTs). These samples have the lowest value of SD and RSD for each of the determined electrochemical parameters. The phase angle values presented in Bode plots (
Figure 3b,
Table A1) recorded in the lowest frequency (0.1 Hz) are related to the heterogeneity of the sample surface. The lowest heterogeneity value of the phase angle (86.7 ± 0.2°) was observed for the 8AuNPs/TNT layers with the smallest gold nanoparticles (Ø: 14.3 ± 1.8 nm). Heterogeneity of the analyzed structures increases with the increase of the gold nanoparticle diameters from 14.3 ± 1.8 nm for 8AuNPs/TNTs, to 28.7 ± 5.2 nm for 80AuNPs/TNTs.
The equivalent circuit allows for good agreement between experimental data and simulated impedance plots for comparative estimation of specific components of the studied surfaces.
The equivalent circuit which corresponds to both the TNT arrays/electrolyte interface and AuNPs/TNTs/electrolyte interface are shown in
Figure 4, where Rs stands for resistance between sample and solution, parallel combination R1Q1 represents resistance and constant phase element with the capacitance C1 of the porous TiO
2, combination R2Q2 determining titanium dioxide nanotubes layer, bare and modified with AuNPs. Due to the TNTs and AuNPs/TNTs surface heterogeneities, a constant phase element Q is used to build a model [
6,
30,
31].
The capacitance values (C1, C2) were calculated according to the equation:
The electrical parameters obtained by fitting equivalent circuits to the measured data are shown in
Table 2. For all electrodes modified by an AuNPs deposition, there is a decrease in charge transfer resistance values when compared with bare TNT, confirming easier electron transfer and presence of deposited gold. The time constant (T) calculated for R2C2 increase with the increased number of deposition cycles, and reaches the maximum value for 60AuNPs/TNTs. The T increase is accompanied by increasing number of gold nanoparticles providing pathways for the electrons. The significant change of time constant recorder for 60AuNPs/TNTs and its further decreasing for 80AuNPs/TNTs indicates more unstable deposition process, i.e., agglomerates formation. Moreover, deposition of AuNPs on TNTs results in the decrease of the Rs, while the biggest change was observed for TNT/AuNPs free of agglomerates, i.e., 8AuNPs/TNTs, 20AuNPs/TNTs, 40AuNPs/TNTs.
As shown in
Table A1,
Table 1 and
Table 2 the lowest value of |Z| was observed for gold nanoparticles deposition in 40 cycles, the lowest RSD for 8, 20, and 40 cycles, OCP close to 0 for 20, and 40 cycles, and minimal values of T providing the best electron transfer for 8, 20, and 40 cycles. That is why for further analysis, including impact assessment of the concentration of tetrachloroauric acid solution on the capacitance and adsorption properties of TNT arrays, the samples 8AuNPs/TNTs, 20AuNPs/TNTs, 40AuNPs/TNTs were selected.
3.2. Characterization of TNTs after AuNPs Deposition—Influence of Various Concentrations of Gold Salt Solutions
Figure 5a–i shows the results of the microscopic analysis of TNTs surface after the AuNPs deposition process carried out at potential ranging from −1.25 V to −0.7 V and for the selected (in the
Section 3.1) number of cycles (8, 20, 40). The cyclic volt amperometry process was carried out in different concentrations of HAuCl
4 solution (1 mM, 5 mM, 10 mM). The samples were denoted correspondingly as xAuNPs/TNTs, where x = 8, 20, 40 is the number of deposition process cycles.
As can be seen in the microphotographs, the Au nanoparticles were spherical and highly dispersed both outside and inside the surface of TNTs, especially on the top of the nanotubes. Due to high current densities, nucleation mostly takes place at the boundaries between the titanium dioxide nanotubes, which is consistent with the results obtained by Bai et al. [
19] and Yang et al. [
27]. The amount of AuNPs loaded on TNT arrays surface increased with the increase of the gold salt solution concentration. However, gold nanoparticles deposited in higher concentration of HAuCl
4 (5 mM, 10 mM) led to production of the nanoparticles with the diameter exceeding the diameter of titanium dioxide nanotubes and formation of many agglomerates (
Figure 5d–i). According to Mahmud et al. [
28], AuNPs deposition on the annealed TNT arrays compared to non-annealed TNT arrays promotes formation of agglomerates around the pore of the titanium dioxide nanotubes. This is due to the removal of residual ions by thermal modification process, which is unfavorable for dispersion of AuNPs and causes its agglomeration [
28]. Additionally, the advantage of using solutions with lower concentration (0.1 mM) is that there is no need of using stabilizers such as polyvinylpyrrolidone [
32], which prevents formation of agglomerates. Thus, in order to obtain well-spherical geometry AuNPs with small diameters, it is necessary to use solutions with lower concentration of tetrachloroauric acid.
According to
Figure 6, linear growth may be observed between the number of CV cycles and the diameter of gold nanoparticles deposited on the TNT arrays in the lower concentration of gold salt solutions: 0.1 mM (
R2 = 1), 1 mM (
R2 = 0.987). For higher concentrations of HAuCl
4, i.e., 5 mM and 10 mM, the diameter of AuNPs decreases logarithmically indicating less stable deposition process.
Table 3 shows the Au components of the surface through EDS analysis in three different places (average value with standard deviation). The results show that the loading amount of Au gradually increases as the concentration of gold salt solution increases, which was confirmed by Babu et al. [
21]. According to Bai et al. [
19], TNT arrays modified with gold nanoparticles provide biocompatible environment favorable for cell attachment, which have a typically elongated morphology with an equal amount of gold of about 4 wt%. Deposition of gold nanoparticles in tetrachloroauric acid solution with various concentrations causes the increase of the nanoparticles diameter (
Table 3). Thus, the obtained results confirmed that it is possible to control and modify the nanoparticle diameters and the deposited gold amount by varying the concentration of electrolyte and deposition cycles. As the concentration of gold salt solution increases, the possibility of nanoparticles aggregation and its heterogeneity (higher value of SD) increases as well. This reduces the ratio of surface area to the volume of the obtained gold nanoparticles.
Table 3 shows the average OCP values for the samples after gold nanoparticles deposition process using cyclic volt amperometry. Just as increasing the number of cycles, the increase of the tetrachloroauric acid concentration also increases the value of open circuit potential to higher positive values. The obtained results are similar to the ones described in the literature. Bai et al. [
19] analyzed TNT arrays after the AuNPs deposition with the use of chronoamperometry method for various time of process. The increase of the amount of the deposited gold causes the increase of the titanium dioxide nanotubes corrosion resistance. High value of open circuit potential was observed for the samples modified with the gold nanoparticles deposited in the solutions of higher concentration (10 mM for 20AuNPs/TNTs and 40AuNPs/TNTs). Electrostatic attraction of oppositely charged protein residues and the electrode surface facilitates the immobilization of the protein in an electro active orientation, further facilitating direct electron transfer between a redox center and the electrode. The application of a potential difference on the electrode can affect the behavior of the proteins on the surface and even its denaturation. For that reason, the electrode potential should be close to zero.
The electrical parameters obtained by fitting equivalent circuits are shown in
Table 4. The T calculated for the lower concentration of HAuCl
4 (0.1 mM, 1 mM) is characterized by minimum value, providing good electron transfer and repeatability of deposition process. Increasing the concentration of HAuCl
4 results in agglomeration formation and less stability of deposition process confirming the higher value of T. For all electrodes modified by AuNPs deposition, the Rs increased with the increase of concentration of the gold salt solution. Among the analyzed samples, the 40AuNPs/TNTs deposition in 0.1 mM of gold salt solution is characterized by the lowest Rs, confirming the best electrical conductivity of this sample.
Performing a cyclic voltammetry process, in which 0.1 mM solution of tetrachloroauric acid was used as the precursor, leads to the production of small diameter gold nanoparticles and prevents the formation of agglomerates. The homogeneity of the AuNPs/TNTs results in higher repeatability expressed by the lowest values of the relative standard deviations for deposition carried out in 0.1 mM HAuCl
4 (
Table A1 and
Table A2). From these samples, 40AuNPs/TNTs is characterized by the lowest Rs and one of the easiest charge transfers (
Table 4). This sample is characterized by positive stationary potential (25 ± 8.6 mV) with the value close to 0 V (
Table 3), which does not deactivate and promotes protein adsorption [
29]. Therefore, for further analysis including XPS and the deposition of biological elements i.e., bovine serum albumin, TNT arrays were chosen deposition of gold nanoparticles by cyclic voltammetry method carried out for 40 cycles in 0.1 mM HAuCl
4.
The results of the XPS analysis of the TNTs before and after modification with gold nanoparticles (40 cycles, 0.1 mM HAuCl
4) are shown in
Figure 7. TiO
2 and Ti
2O
3 were found on the surface of the nanotube layers. The standard binding energy of Ti 2
p3/2 in TiO
2 for Ti
3+ is usually located at 457.7 eV and for Ti
4+ is at 459.5 eV [
33,
34]. The O 1
s binding energy for TiO
2 is 529.3 eV [
34]. The analysis of the XPS depth profile of TNTs and 40AuNPs/TNTs indicates higher amount of oxygen absorbed inside of the oxide film rather than on its surface. Thermal modification of TNTs results in the occurrence of the lack of oxygen on the surface, which proves its deficiency, the presence of oxygen vacancies and results in improved TNTs electrical conductivity [
25]. For 40AuNPs/TNTs, the main 4
f7/2 line is shifted to lower binding energy (83.2 eV), which was found to occur in case of nanoparticles of gold with well spherical geometry [
35]. This shift is caused by the initial state effects where spherical NPs have larger fraction of uncoordinated surface atoms reducing their binding energies relative to nanoparticles with large diameter [
35].
3.3. Evaluation of Experimental Conditions for BSA Protein Adsorption on the TNT and 40AuNPs Arrays
Figure 8 shows the results of the electrochemical analysis in Nyquist representation for thermal modification of the TNTs and the AuNPs/TNTs before and after serum bovine albumin immobilization. The immobilization procedure was executed in accordance with Kopac et al. [
36], in which BSA deposition was carried out on a double-walled, carbon nanotube (DWCNT) system. For this study, the optimum experimental conditions were observed to be at 40 °C due to the highest value of adsorption efficiency (72%) compared to other temperatures: 25 °C (27%), 30 °C (32%), 37 °C (53%). This showed that the adsorbate onto DWCNT increased with increasing temperature, which, according to [
36], can be attributed to the availability of more adsorption sites and increase in the sorptive surface area.
The values of electrochemical parameters presented in
Figure 8 obtained for the 40AuNPs/TNTs and the TNT layers before and after the deposition of BSA show that the highest increase in impedance modulus recorded in the lowest frequency (0.1 Hz) by 1247 Ω (28%) was observed for samples modified with gold nanoparticles, while for non-modified titanium dioxide nanotubes, the increase was only 843 Ω (14%).
The electrical parameters obtained by fitting equivalent circuits are shown in
Table 5. The equivalent circuit is shown in
Figure 4. Deposition of BSA on TNTs and 40AuNPs/TNTs arrays causes an increase in the Rs value. The highest increase in Rs value of 16.29 Ω was recorded for the samples after modification with gold nanoparticles, which confirms the increased adsorption of bovine serum albumin for this platform. For 40AuNPs/TNTs arrays after the BSA deposition process, they are characterized by almost double increase of the T value, which is caused by the increase of electron transfer resistance due to the formation of a protein layer.
Better adsorption of biological elements on the 40AuNPs/TNT arrays results from an increase in the direction of positive OCP values caused by modification of gold nanoparticles. It is very important for the adsorption of BSA, which in the PBS solution (pH 7.4) has negative charge (isoelectric point of BSA protein −5.4). According to several articles on protein immobilization, an appropriate pH for the termination of proteins would be around 7 to 8 [
37,
38]. During the formation of biological layer on the surface, each adsorbing molecule must go through the following steps: transport toward the surface, attachment, and another spreading on the surface. According to the electrostatic binding hypothesis, the attraction between the negative surface residues due to the isoelectric point of BSA and the positive charge from the surface are responsible for the strong binding of BSA to gold nanoparticles. In this hypothesis, the protein attaches itself to the passivation layer on the gold surface, with little direct interaction between BSA and the gold surface [
38]. According to Liu [
39] modification of electrode surfaces with gold nanoparticles provides a microenvironment similar to that of proteins in native systems, and gives the protein molecules orientation freedom. Peng et al. confirmed that the highest titanium dioxide nanotubes promote adsorption and stability of BSA protein binding [
40].
 —the lowest value of RSD.
—the lowest value of RSD. —the lowest value of RSD
—the lowest value of RSD