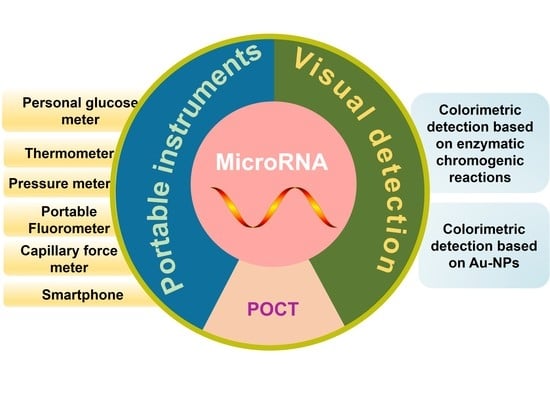
Advances in Point-of-Care Testing of microRNAs Based on Portable Instruments and Visual Detection
Abstract
:1. Introduction
2. POCT of miRNAs
2.1. POCT of miRNAs Based on Portable Instruments
2.1.1. miRNA Detection Based on a Personal Glucose Meter
2.1.2. Detection of miRNAs Based on a Thermometer
2.1.3. Detection of miRNAs Based on a Pressure Meter
2.1.4. Detection of miRNAs Based on Portable Fluorometers
2.1.5. Detection of miRNAs Based on a Capillary Force Meter

2.1.6. Detection of miRNAs Based on Smartphones

| Methods | miRNA | Detection Limit | Samples | Time | Reference |
|---|---|---|---|---|---|
| Personal glucose meter | miR-21 | 0.41 nM/ 1 million cells | synthesized miR-21/A549 cell lysates | <2 h | [21] |
| miR-21 | 10 fM | synthesized miR-21 | <2 h | [23] | |
| miR-21 miRNA205 | 2.4 pM 1.1 pM | synthesized miR-21 synthesized miRNA205 | <3 h | [26] | |
| miR-21 | 3.65 nM | synthesized miR-21 clinical serum samples from cancer patients | 2 h | [22] | |
| miR-21 | 60 pM 3 × 106 cells/mL | synthesized miR-21 MCF-7, A549 and HeLa cell lysates | <3 h | [24] | |
| miR-21 | 68.08 fM | synthesized miR-21 urine samples from DIKI mice | 1.5 h | [25] | |
| miRNA-155 | 0.36 fM | synthesized miRNA-155 | >5 h | [27] | |
| miR-21, miR-335, miR-155, and miR-122 | 0.325 fmol | synthesized miRNAs extract from HeLa, HepG2, MCF-7, and L02 Cells | 6 h | [28] | |
| Thermometer | miR-21 | 7.8 nM | synthesized miR-21 HeLa cell lysate | Not mentioned | [35] |
| miRNA-141 | 0.5 pM | synthesized miRNA-141 | >8 h | [36] | |
| Pressure meter | miR-21 | 7.6 fM 100 cells | synthesized miR-21 A549, MCF-7, HepG2 and HL-7702 cells | 20 min | [38] |
| miR-21 | 10 pM | Serum | 0.5 h | [39] | |
| Portable fluorometer | miR-574-5p | 2 ng/μL | RNA extract from 5XFAD mice | >3 h | [40] |
| Capillary force meter | miR-21 | 10 nM | Human serum | 1 h | [43] |
| miR-21 | MCF-7 cell line | 25 min | [44] | ||
| Smartphone | miR-133a | 0.3 pM | synthesized miR-133a in serum | >5 h | [50] |
| miRNA-499, miRNA-133a | 10 fM | synthesized miR-133a in serum | 13 h | [51] | |
| let-7a | 1.7 fM | synthesized let-7a human serum | 2.75 h | [56] | |
| miR-133a, miR-499 | 1 fM | Synthesized miRNAs human serum | - | [52] | |
| miR-21, let-7a | fM | Synthesized miRNAs human serum | <2 h | [49] | |
| miR-21 | 1.43 pM | Synthesized miR-21 human serum, urine | 0.5 h | [58] | |
| miR-224 | 1.6 fM | Synthesized miR-224 human plasma | <4.5 h | [59] | |
| miR-21 | 100 fM 500 cells | Synthesized miR-21 MCF-7 and L02 cells | >1 h | [57] |
2.2. Visual Detection of miRNAs Based on Colorimetry
2.2.1. Colorimetric Detection Based on Au-NPs

2.2.2. Colorimetric Method Based on Enzymatic Chromogenic Reactions

| Methods | miRNA | Detection Limit | Samples | Time | Reference |
|---|---|---|---|---|---|
| Colorimetric detection based on Au-NPs | miR-21 miR-155 | 5 ng μL−1 | Plasma | <3 min | [63] |
| miR-93 miR-223 | - | Human serum | - | [64] | |
| miR-34a miR-210 | 50 ng μL−1 | Urine | <20 min | [66] | |
| miR-195 | 40 fM | Human serum | 10 min | [67] | |
| miR-210-3p | 10 pM | Urine | 20 min | [68] | |
| miR-21 miR-155 | 1 ng μL−1 | Multiple cancerous cell lines and primary fibroblast | <10 min | [69] | |
| miR-21 miR-141 | 3 pM | Synthesized miRNA human serum samples | <5 h | [70] | |
| miR-137 | 0.5 nM | Plasma | 1 min | [72] | |
| miR-146a | 5 nM | Raw cow milk | 20 min | [76] | |
| let-7a | 0.13 pM | A549 cells | 50 min | [81] | |
| miR-148a | 1.9 nM | Plasma | 5 min | [75] | |
| miR-122 | 16 pM | Cancerous cell lines | 2 h | [79] | |
| let-7a | 3.13 fM | Human serum | 1 h | [80] | |
| miR-203 | 10 pM | MCF-7 cells | - | [82] | |
| miR-21 | 0.23 fM | HeLa, MCF-7, AGS cells | 0.5 h | [84] | |
| let-7a | 4.176 aM | Synthesized let-7a | 1 h | [83] | |
| miR-221-3p | 46 fM | BEL-7404, MDA-MB231, HeLa, and 22Rv1cells | 1 h | [85] | |
| miR-143 | 1 fM | Synthesized miR-143 Prostate cancer cell lines VCaP, LNCaP, Du145, and PC-3 | >1.5 h | [87] | |
| Colorimetric detection based on enzymatic chromogenic reactions | let-7a | 7.4 fM | Synthesized let-7a | 2.5 h | [92] |
| miR-122 | 0.15 aM | Serum | 5 min | [93] | |
| miR-21 | 0.2 pM | Serum | 50 min | [94] | |
| miR-21 | 1 aM | Serum | <4 h | [95] | |
| Let-7a | 34 fM | A549 cells | 4 h | [96] | |
| miR-10b | 1 fM | Serum and cell extracts | 20 min | [97] | |
| miR-141 | 0.48 nM | Serum | >3 h | [100] | |
| miR-21 | 1 pM | Serum | 150 min | [102] | |
| miR-141 | 0.5 pM | Prostate cancer cells | 210 min | [104] | |
| miR-21 | 90.3 fM | Serum | <1.5 h | [105] | |
| miR-21, miR-17 | 1.7 fM | MCF-7 | 80 min | [106] | |
| let-7a | 0.1 nM | Serum | 3 min | [108] | |
| miR-21 | 44.76 fM | Exosome | 2 h | [111] | |
| miR-21, miR-155 | 0.38 nM | Blood | >1 h | [112] | |
| miR-21 | 4.5 nM | MCF-7 and serum | 130 min | [113] | |
| miR-21 | 5 fM | Plasma sample Cancer cells Tumor tissues | >6.5 h | [109] | |
| miR-155 | 0.6 pM | Plasma | 15 min | [115] | |
| miR-205, miR-944 | 36.4 fM | Serum | >2 h | [116] | |
| miR-155 | 31.8 fM | Serum | 1 h | [117] | |
| miR-223 miR-143 | 20 pM | Synthesized miR-223 iPSCs and CMs | 3.5 h | [118] |
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Brate, J.; Neumann, R.S.; Fromm, B.; Haraldsen, A.A.B.; Tarver, J.E.; Suga, H.; Donoghue, P.C.J.; Peterson, K.J.; Ruiz-Trillo, I.; Grini, P.E.; et al. Unicellular Origin of the Animal MicroRNA Machinery. Curr. Biol. 2018, 28, 3288–3295.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Higa, G.S.; de Sousa, E.; Walter, L.T.; Kinjo, E.R.; Resende, R.R.; Kihara, A.H. MicroRNAs in neuronal communication. Mol. Neurobiol. 2014, 49, 1309–1326. [Google Scholar] [CrossRef]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol. Biol. 2017, 1509, 1–10. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, L.; Zhang, J.; Zhao, Y.; Li, Z. Recent advances in microRNA detection. Analyst 2018, 143, 1758–1774. [Google Scholar] [CrossRef]
- Luppa, P.B.; Muller, C.; Schlichtiger, A.; Schlebusch, H. Point-of-care testing (POCT): Current techniques and future perspectives. Trends Anal. Chem. 2011, 30, 887–898. [Google Scholar] [CrossRef]
- Wei, H.; Peng, Y.; Bai, Z.; Rong, Z.; Wang, S. Duplex-specific nuclease signal amplification-based fluorescent lateral flow assay for the point-of-care detection of microRNAs. Anal. 2021, 146, 558–564. [Google Scholar] [CrossRef]
- Giuffrida, M.C.; Spoto, G. Integration of isothermal amplification methods in microfluidic devices: Recent advances. Biosens. Bioelectron. 2017, 90, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Qin, S.; Wang, Q.; Yang, X.; Wang, K. Optical fiber amplifier for quantitative and sensitive point-of-care testing of myoglobin and miRNA-141. Biosens. Bioelectron. 2019, 129, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Lu, F.; Wang, J.; Wang, K.; Liu, B.; Li, N.; Tang, B. A portable point-of-care testing system to diagnose lung cancer through the detection of exosomal miRNA in urine and saliva. Chem. Commun. 2020, 56, 8968–8971. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, D.P.; Kalligosfyri, P.M.; Kyriakou, I.K.; Christopoulos, T.K. Advances in microRNA analysis. Anal. Bioanal. Chem. 2018, 410, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Gines, G.; Menezes, R.; Xiao, W.; Rondelez, Y.; Taly, V. Emerging isothermal amplification technologies for microRNA biosensing: Applications to liquid biopsies. Mol. Asp. Med. 2020, 72, 100832. [Google Scholar] [CrossRef]
- Jet, T.; Gines, G.; Rondelez, Y.; Taly, V. Advances in multiplexed techniques for the detection and quantification of microRNAs. Chem. Soc. Rev. 2021, 50, 4141–4161. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, J.; Xiao, B.; Sun, X.; Xie, R.; Chen, A. Recent advances in the rapid detection of microRNA with lateral flow assays. Biosens. Bioelectron. 2022, 211, 114345. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Amin Mahdian, S.M.; Ebrahimi, M.S.; Taghizadieh, M.; Vosough, M.; Sadri Nahand, J.; Hosseindoost, S.; Vousooghi, N.; Javar, H.A.; Larijani, B.; et al. Microfluidics for detection of exosomes and microRNAs in cancer: State of the art. Mol. Ther. Nucleic Acids 2022, 28, 758–791. [Google Scholar] [CrossRef]
- Ahn, J.K.; Kim, H.Y.; Park, K.S.; Park, H.G. A Personal Glucose Meter for Label-Free and Washing-Free Biomolecular Detection. Anal. Chem. 2018, 90, 11340–11343. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 2011, 3, 697–703. [Google Scholar] [CrossRef]
- Wu, T.; Yang, Y.; Cao, Y.; Song, Y.; Xu, L.P.; Zhang, X.; Wang, S. Bioinspired DNA-Inorganic Hybrid Nanoflowers Combined with a Personal Glucose Meter for Onsite Detection of miRNA. ACS Appl. Mater. Interfaces 2018, 10, 42050–42057. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Cai, R.; Gui, S.; Zhang, Y.; Wang, X.; Zhou, N. A portable and quantitative detection of microRNA-21 based on cascade enzymatic reactions with dual signal outputs. Talanta 2021, 235, 122802. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, H.; Zhao, R.; Tang, Y.; Li, B. Sensitive, general and portable detection of RNAs combining duplex-specific nuclease transduction with an off-shelf signalling platform. Chem. Commun. 2021, 57, 5714–5717. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cao, Y.; Yang, Y.; Zhang, X.; Wang, S.; Xu, L.P.; Zhang, X. A three-dimensional DNA walking machine for the ultrasensitive dual-modal detection of miRNA using a fluorometer and personal glucose meter. Nanoscale 2019, 11, 11279–11284. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Lu, M.; Zhang, W.; Xu, Z.; Yu, B.Y.; Tian, J. Point-of-care testing of MicroRNA based on personal glucose meter and dual signal amplification to evaluate drug-induced kidney injury. Anal. Chim. Acta 2020, 1112, 72–79. [Google Scholar] [CrossRef]
- Gong, S.; Li, J.; Pan, W.; Li, N.; Tang, B. Duplex-Specific Nuclease-Assisted CRISPR-Cas12a Strategy for MicroRNA Detection Using a Personal Glucose Meter. Anal. Chem. 2021, 93, 10719–10726. [Google Scholar] [CrossRef]
- Fu, P.; Xu, M.; Xing, S.; Zhao, Y.; Zhao, C. Dual cascade isothermal amplification reaction based glucometer sensors for point-of-care diagnostics of cancer-related microRNAs. Anal. 2021, 146, 3242–3250. [Google Scholar] [CrossRef]
- Si, Y.; Li, L.; Wang, N.; Zheng, J.; Yang, R.; Li, J. Oligonucleotide Cross-Linked Hydrogel for Recognition and Quantitation of MicroRNAs Based on a Portable Glucometer Readout. ACS Appl. Mater. Interfaces 2019, 11, 7792–7799. [Google Scholar] [CrossRef]
- Doe, E.; Hayth, H.L.; Brumett, R.; Khisamutdinov, E.F. Effective, Rapid, and Small-Scale Bioconjugation and Purification of "Clicked" Small-Molecule DNA Oligonucleotide for Nucleic Acid Nanoparticle Functionalization. Int. J. Mol. Sci. 2023, 24, 4797. [Google Scholar] [CrossRef]
- He, Q.; Wu, Q.; Feng, X.; Liao, Z.; Peng, W.; Liu, Y.; Peng, D.; Liu, Z.; Mo, M. Interfacing DNA with nanoparticles: Surface science and its applications in biosensing. Int. J. Biol. Macromol. 2020, 151, 757–780. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, H.; Lu, Y. Translating molecular detections into a simple temperature test using a target-responsive smart thermometer. Chem. Sci. 2018, 9, 3906–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Luo, F.; Lin, Y.; Dong, N.; Li, C.; Lin, Z. Quantitative gold nanorods based photothermal biosensor for glucose using a thermometer as readout. Talanta 2021, 230, 122364. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Tao, Y.; Shen, H.; Yang, C.; Tian, T.; Yang, L.; Zhu, Z. A polypyrrole-mediated photothermal biosensor with a temperature and pressure dual readout for the detection of protein biomarkers. Analyst 2022, 147, 2671–2677. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lin, S.; Li, Y.; Cheng, C.; Han, X. Near-infrared photothermal immunoassay for pancreatic cancer biomarker CA 19-9 on a digital thermometer. Anal. Chim. Acta 2020, 1098, 117–124. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Chen, Z.; Cui, J.; Yang, L.; Lu, Z.; Qi, F.; Wang, H. Photothermal Detection of MicroRNA Using a Horseradish Peroxidase-Encapsulated DNA Hydrogel With a Portable Thermometer. Front. Bioeng. Biotechnol. 2021, 9, 799370. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Li, L.; Tan, X.; Ge, S.; Zhang, L.; Yu, J.; Zhang, Y. Photothermal-Reagent-Triggered Visual Thermoresponsive and Quantized Photoelectrochemical Dual-Signal Assay. ACS Sens. 2022, 7, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Guan, Z.; Liu, D.; Jia, S.; Li, J.; Lei, Z.; Lin, S.; Ji, T.; Tian, Z.; Yang, C.J. Translating Molecular Recognition into a Pressure Signal to enable Rapid, Sensitive, and Portable Biomedical Analysis. Angew. Chem. Int. Ed. Engl. 2015, 54, 10448–10453. [Google Scholar] [CrossRef]
- Shi, L.; Lei, J.; Zhang, B.; Li, B.; Yang, C.J.; Jin, Y. Ultrasensitive and Facile Detection of MicroRNA via a Portable Pressure Meter. ACS Appl. Mater. Interfaces 2018, 10, 12526–12533. [Google Scholar] [CrossRef]
- Shi, L.; Liu, W.; Li, B.; Yang, C.J.; Jin, Y. Multichannel Paper Chip-Based Gas Pressure Bioassay for Simultaneous Detection of Multiple MicroRNAs. ACS Appl. Mater. Interfaces 2021, 13, 15008–15016. [Google Scholar] [CrossRef]
- Nambannor Kunnath, R.; Venukumar, A.; Gorthi, S.S. Handheld fluorometer for in-situ melamine detection via interference synthesis of dsDNA-templated copper nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 235, 118304. [Google Scholar] [CrossRef]
- Lim, J.; Kim, S.; Oh, S.J.; Han, S.M.; Moon, S.Y.; Kang, B.; Seo, S.B.; Jang, S.; Son, S.U.; Jung, J.; et al. miRNA sensing hydrogels capable of self-signal amplification for early diagnosis of Alzheimer’s disease. Biosens. Bioelectron. 2022, 209, 114279. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiao, X.; Du, X.; Wang, F.; Wei, Q.; Wen, Y.; Zhang, X. Wettability alteration in a functional capillary tube for visual quantitative point of care testing. Analyst 2018, 143, 3001–3005. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Men, X.; Gao, G.; Tian, Y.; Wen, Y.; Zhang, X. A distance-based capillary biosensor using wettability alteration. Lab. A Chip 2021, 21, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.H.; Li, Y.S.; Jiang, C.; Chen, D.S.; Wen, Y.Q.; Li, Z.P. Capillarity self-driven DNA hydrogel sensor for visual quantification of microRNA. Sens. Actuat. B-Chem. 2020, 313, 128036. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017-2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Kwon, L.; Long, K.D.; Wan, Y.; Yu, H.; Cunningham, B.T. Medical diagnostics with mobile devices: Comparison of intrinsic and extrinsic sensing. Biotechnol. Adv. 2016, 34, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Kanchi, S.; Sabela, M.I.; Mdluli, P.S.; Inamuddin; Bisetty, K. Smartphone based bioanalytical and diagnosis applications: A review. Biosens. Bioelectron. 2018, 102, 136–149. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, L.; Wang, H.; Ji, W.; Zhang, Z.; Zhang, Y.; Yang, Z.; Cao, Z.; Zhang, S.; Chang, J. Intelligent Detection Platform for Simultaneous Detection of Multiple MiRNAs Based on Smartphone. ACS Sens. 2019, 4, 1873–1880. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, L.; Wang, Q.; Mi, L.; Li, T. Spherical Nucleic Acid Enzyme (SNAzyme) Boosted Chemiluminescence miRNA Imaging Using a Smartphone. Anal. Chem. 2019, 91, 3652–3658. [Google Scholar] [CrossRef]
- Shi, L.; Sun, Y.; Mi, L.; Li, T. Target-Catalyzed Self-Growing Spherical Nucleic Acid Enzyme (SNAzyme) as a Double Amplifier for Ultrasensitive Chemiluminescence MicroRNA Detection. ACS Sens. 2019, 4, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Sun, Y.; Shi, L.; Li, T. Hemin-Bridged MOF Interface with Double Amplification of G-Quadruplex Payload and DNAzyme Catalysis: Ultrasensitive Lasting Chemiluminescence MicroRNA Imaging. ACS Appl. Mater. Interfaces 2020, 12, 7879–7887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, S.; Liu, B.; Wang, C.; Huang, Y.; Zhang, X.; Zhang, S. Chemiluminescence Sensor for miRNA-21 Detection Based on CRISPR-Cas12a and Cation Exchange Reaction. Anal. Chem. 2023, 95, 3332–3339. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Kim, K.T.; Lindberg, E.; Winssinger, N. Smartphone DNA or RNA Sensing Using Semisynthetic Luciferase-Based Logic Device. ACS Sens. 2020, 5, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, L.; Yang, L.; Ni, W.; Li, Y.; Wu, Y. Tandem reassembly of split luciferase-DNA chimeras for bioluminescent detection of attomolar circulating microRNAs using a smartphone. Biosens. Bioelectron. 2021, 173, 112824. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Ni, W.; Luo, Q.; Zhu, C.; Wu, Y. Portable and Field-Ready Detection of Circulating MicroRNAs with Paper-Based Bioluminescent Sensing and Isothermal Amplification. Anal. Chem. 2019, 91, 14838–14841. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Wang, Y.; Song, S.; Chen, C.; Yao, B.; Wang, M. A fishhook probe-based rolling circle amplification (FP-RCA) assay for efficient isolation and detection of microRNA without total RNA extraction. Analytical 2018, 143, 5046–5053. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Cui, X.; Yang, L.; Zhang, J.; Mao, X.; Gao, Y. Sensitive detection of miRNA based on enzyme-propelled multiple photoinduced electron transfer strategy. Mikrochim. Acta 2021, 188, 219. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Xie, H.; Liu, H.; Liu, S.; He, D.; Mi, P.; He, S.; Wang, J.; Sun, Y. NIR-to-Vis Handheld Platforms for Detecting miRNA Level and Mutation Based on Sub-10 nm Sulfide Nanodots and HCR Amplification. ACS Appl. Mater. Interfaces 2022, 14, 10212–10226. [Google Scholar] [CrossRef]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Hosokawa, K.; Maeda, M. Rapid aggregation of gold nanoparticles induced by non-cross-linking DNA hybridization. J. Am. Chem. Soc. 2003, 125, 8102–8103. [Google Scholar] [CrossRef] [PubMed]
- Mollasalehi, H.; Hamidi, A. Early-phase nano-genosensing of cell-free nucleobiomarkers in the plasma of cancerous patients. Nanomedicine 2021, 32, 102344. [Google Scholar] [CrossRef] [PubMed]
- Pitou, M.; Papi, R.M.; Tzavellas, A.N.; Choli-Papadopoulou, T. ssDNA-Modified Gold Nanoparticles as a Tool to Detect miRNA Biomarkers in Osteoarthritis. ACS Omega 2023, 8, 7529–7535. [Google Scholar] [CrossRef]
- Baptista, P.; Doria, G.; Henriques, D.; Pereira, E.; Franco, R. Colorimetric detection of eukaryotic gene expression with DNA-derivatized gold nanoparticles. J. Biotechnol. 2005, 119, 111–117. [Google Scholar] [CrossRef]
- Nossier, A.I.; Shehata, N.I.; Morsy, S.M.; Saeed, D.F.; Elsayed, N.M.; Ismail, M.F.; Eissa, S. Determination of certain urinary microRNAs as promising biomarkers in diabetic nephropathy patients using gold nanoparticles. Anal. Biochem. 2020, 609, 113967. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, K.; Du, Z.; Xin, J.; Luo, M.; Wang, F. Colorimetric identification of miRNA-195 sequence for diagnosing osteosarcoma. Biotechnol. Appl. Biochem. 2022, 69, 974–980. [Google Scholar] [CrossRef]
- Nossier, A.I.; Abdelzaher, H.; Matboli, M.; Eissa, S. Dual approach for the colorimetric determination of unamplified microRNAs by using citrate capped gold nanoparticles. Mikrochim. Acta 2018, 185, 236. [Google Scholar] [CrossRef]
- Mollasalehi, H.; Shajari, E. A colorimetric nano-biosensor for simultaneous detection of prevalent cancers using unamplified cell-free ribonucleic acid biomarkers. Bioorg Chem. 2021, 107, 104605. [Google Scholar] [CrossRef]
- Li, Z.H.; Yang, M.; Zhao, C.X.; Shu, Y. Bifunctional Y-shaped probe combined with dual amplification for colorimetric sensing and molecular logic operation of two miRNAs. Talanta 2023, 259, 124480. [Google Scholar] [CrossRef]
- Tian, R.; Zheng, X. Sensitive Colorimetric Detection of MicroRNA Based on Target Catalyzed Double-arm Hairpin DNA Assembling. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2016, 32, 751–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delkhahi, S.; Rahaie, M.; Rahimi, F. Design and Fabrication a Gold Nanoparticle-DNA Based Nanobiosensor for Detection of microRNA Involved in Alzheimer’s Disease. J. Fluoresc. 2017, 27, 603–610. [Google Scholar] [CrossRef]
- Miao, J.; Wang, J.; Guo, J.; Gao, H.; Han, K.; Jiang, C.; Miao, P. A plasmonic colorimetric strategy for visual miRNA detection based on hybridization chain reaction. Sci. Rep. 2016, 6, 32219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Brook, M.A.; Li, Y. Design of gold nanoparticle-based colorimetric biosensing assays. Chembiochem A Eur. J. Chem. Biol. 2008, 9, 2363–2371. [Google Scholar] [CrossRef]
- Cai, J.; Ding, L.; Gong, P.; Huang, J. A colorimetric detection of microRNA-148a in gastric cancer by gold nanoparticle-RNA conjugates. Nanotechnology 2020, 31, 095501. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Visedo, A.; Gallego, B.; Royo, L.J.; Soldado, A.; Valledor, M.; Ferrero, F.J.; Campo, J.C.; Costa-Fernandez, J.M.; Fernandez-Arguelles, M.T. Visual detection of microRNA146a by using RNA-functionalized gold nanoparticles. Mikrochim. Acta 2020, 187, 192. [Google Scholar] [CrossRef]
- Oishi, M.; Sugiyama, S. An Efficient Particle-Based DNA Circuit System: Catalytic Disassembly of DNA/PEG-Modified Gold Nanoparticle-Magnetic Bead Composites for Colorimetric Detection of miRNA. Small 2016, 12, 5153–5158. [Google Scholar] [CrossRef]
- Park, J.; Yeo, J.S. Colorimetric detection of microRNA miR-21 based on nanoplasmonic core-satellite assembly. Chem. Commun. 2014, 50, 1366–1368. [Google Scholar] [CrossRef]
- Wang, Q.; Li, R.D.; Yin, B.C.; Ye, B.C. Colorimetric detection of sequence-specific microRNA based on duplex-specific nuclease-assisted nanoparticle amplification. Analyst 2015, 140, 6306–6312. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, Y.Q.; Ding, Y.; Feng, N.; Li, M.; Yin, Y. Engineering entropy-driven based multiple signal amplification strategy for visualized assay of miRNA by naked eye. Talanta 2021, 235, 122810. [Google Scholar] [CrossRef]
- Hu, B.; Guo, J.; Xu, Y.; Wei, H.; Zhao, G.; Guan, Y. A sensitive colorimetric assay system for nucleic acid detection based on isothermal signal amplification technology. Anal. Bioanal. Chem. 2017, 409, 4819–4825. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shang, X.; Liu, J.; Wang, Y.; Guo, Y.; You, J. A universal colorimetry for nucleic acids and aptamer-specific ligands detection based on DNA hybridization amplification. Anal. Biochem. 2017, 528, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, B.; Zhang, C.; Guan, Y. A Novel Design Combining Isothermal Exponential Amplification and Gold-Nanoparticles Visualization for Rapid Detection of miRNAs. Int. J. Mol. Sci. 2018, 19, 3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, S.; Chen, G.; Jia, X.; Mao, X.; Chen, T.; Mao, D.; Zhang, W.; Xiong, W. Exponential amplification reaction and triplex DNA mediated aggregation of gold nanoparticles for sensitive colorimetric detection of microRNA. Anal. Chim. Acta 2020, 1095, 179–184. [Google Scholar] [CrossRef]
- Li, R.D.; Yin, B.C.; Ye, B.C. Ultrasensitive, colorimetric detection of microRNAs based on isothermal exponential amplification reaction-assisted gold nanoparticle amplification. Biosens. Bioelectron. 2016, 86, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Tian, T.; Sun, J.; Hu, M.; Wang, X.; Xiong, E.; Cheng, M.; Bao, Y.; Lin, W.; Jiang, J.; et al. Universal and Naked-Eye Gene Detection Platform Based on the Clustered Regularly Interspaced Short Palindromic Repeats/Cas12a/13a System. Anal. Chem. 2020, 92, 4029–4037. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, Z.; Lu, J.; Ren, X.; Ma, Y. Ultrasensitive visual detection of miRNA-143 using a CRISPR/Cas12a-based platform coupled with hyperbranched rolling circle amplification. Talanta 2023, 251, 123784. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Liu, C.; Gou, S.; Hu, S.; Guo, W. A portable colorimetric point-of-care testing platform for MicroRNA detection based on programmable entropy-driven dynamic DNA network modulated DNA-gold nanoparticle hybrid hydrogel film. Biosens. Bioelectron. 2023, 225, 115073. [Google Scholar] [CrossRef]
- Shahsavar, K.; Hosseini, M.; Shokri, E.; Xu, G. New insight into G-quadruplexes; diagnosis application in cancer. Anal. Biochem. 2021, 620, 114149. [Google Scholar] [CrossRef]
- Cao, Y.; Li, W.; Pei, R. Exploring the catalytic mechanism of multivalent G-quadruplex/hemin DNAzymes by modulating the position and spatial orientation of connected G-quadruplexes. Anal. Chim. Acta 2022, 1221, 340105. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q.; Lang, Y.; Jiang, X.; Wu, P. Rationale of 3,3’,5,5’-Tetramethylbenzidine as the Chromogenic Substrate in Colorimetric Analysis. Anal. Chem. 2020, 92, 12400–12406. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Wang, H.; Wu, J.; Zhu, F.; Zou, P. Label-free and enzyme-free colorimetric detection of microRNA by catalyzed hairpin assembly coupled with hybridization chain reaction. Biosens. Bioelectron. 2016, 81, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Qi, T.; Yao, Y.; Tang, L.; Chen, W.; Chen, T.; Shen, W.; Kong, D.; Shi, H.W.; Liu, T.; et al. Magnetic Three-Phase Single-Drop Microextraction for Rapid Amplification of the Signals of DNA and MicroRNA Analysis. Anal. Chem. 2020, 92, 12290–12296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, C.; Luan, C.; Gao, P.; Wang, H.; Chi, J.; Kong, T. Distance-based quantification of miRNA-21 by the coffee-ring effect using paper devices. Mikrochim. Acta 2020, 187, 513. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Ravan, H.; Mohammadi, A.; Mohammad-Rezaei, R.; Norouzi, A.; Hosseinzadeh, H. Target-triggered three-way junction in conjugation with catalytic concatemers-functionalized nanocomposites provides a highly sensitive colorimetric method for miR-21 detection. Biosens. Bioelectron. 2018, 117, 567–574. [Google Scholar] [CrossRef]
- Li, R.; Liu, Q.; Jin, Y.; Li, B. Sensitive colorimetric determination of microRNA let-7a through rolling circle amplification and a peroxidase-mimicking system composed of trimeric G-triplex and hemin DNAzyme. Mikrochim. Acta 2020, 187, 139. [Google Scholar] [CrossRef]
- Zhou, T.; Huang, M.; Lin, J.; Huang, R.; Xing, D. High-Fidelity CRISPR/Cas13a trans-Cleavage-Triggered Rolling Circle Amplified DNAzyme for Visual Profiling of MicroRNA. Anal. Chem. 2021, 93, 2038–2044. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, Y.; Mao, X.; Wei, Y.; Song, H.; Chen, N.; Huang, Q.; Fan, C.; Li, D. DNAzyme-based rolling-circle amplification DNA machine for ultrasensitive analysis of microRNA in Drosophila larva. Anal. Chem. 2012, 84, 7664–7669. [Google Scholar] [CrossRef]
- Li, D.; Cheng, W.; Yan, Y.; Zhang, Y.; Yin, Y.; Ju, H.; Ding, S. A colorimetric biosensor for detection of attomolar microRNA with a functional nucleic acid-based amplification machine. Talanta 2016, 146, 470–476. [Google Scholar] [CrossRef]
- Park, Y.; Lee, C.Y.; Kang, S.; Kim, H.; Park, K.S.; Park, H.G. Universal, colorimetric microRNA detection strategy based on target-catalyzed toehold-mediated strand displacement reaction. Nanotechnology 2018, 29, 085501. [Google Scholar] [CrossRef]
- Lee, J.; Na, H.K.; Lee, S.; Kim, W.K. Advanced graphene oxide-based paper sensor for colorimetric detection of miRNA. Mikrochim. Acta 2021, 189, 35. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, E.; Ravan, H.; Mohammadi, A.; Pourghadamyari, H. Colorimetric detection of miRNA-21 by DNAzyme-coupled branched DNA constructs. Talanta 2020, 216, 120913. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Park, J.S.; Chun, H.S.; Yoon, S.; Kim, W.K.; Lee, J. A fluorescence/colorimetric dual-mode sensing strategy for miRNA based on graphene oxide. Anal. Bioanal. Chem. 2020, 412, 233–242. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, W.; Li, X.; Chai, Y.; Yuan, R.; Xiang, Y. MicroRNA-triggered, cascaded and catalytic self-assembly of functional "DNAzyme ferris wheel" nanostructures for highly sensitive colorimetric detection of cancer cells. Nanoscale 2015, 7, 9055–9061. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yuan, L.; Xu, Y.; He, B. Target-catalyzed self-assembled spherical G-quadruplex/hemin DNAzymes for highly sensitive colorimetric detection of microRNA in serum. Anal. Chim. Acta 2023, 1247, 340879. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shen, B.; Wang, H.; Sun, X.; Cheng, W.; Zhao, H.; Ju, H.; Ding, S. A novel and versatile nanomachine for ultrasensitive and specific detection of microRNAs based on molecular beacon initiated strand displacement amplification coupled with catalytic hairpin assembly with DNAzyme formation. Anal. 2015, 140, 5469–5474. [Google Scholar] [CrossRef]
- Yan, C.; Jiang, C.; Jiang, J.; Yu, R. Simple, colorimetric detection of microRNA based on target amplification and DNAzyme. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2013, 29, 605–610. [Google Scholar] [CrossRef]
- Xu, H.; Yang, F.; Chen, D.; Ye, W.; Xue, G.; Jia, L. Trigging stepwise-strand displacement amplification lights up numerous G-quadruplex for colorimetric signaling of serum microRNAs. iScience 2023, 26, 106331. [Google Scholar] [CrossRef]
- Zeng, Y.; Yue, H.; Cao, B.; Li, Y.; Yang, M.; Mao, C. Target-Triggered Formation of Artificial Enzymes on Filamentous Phage for Ultrasensitive Direct Detection of Circulating miRNA Biomarkers in Clinical Samples. Angew. Chem. Int. Ed. Engl. 2022, 61, e202210121. [Google Scholar] [CrossRef]
- Deng, H.; Shen, W.; Ren, Y.; Gao, Z. A highly sensitive and selective homogenous assay for profiling microRNA expression. Biosens. Bioelectron. 2014, 54, 650–655. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, T.; Zhang, L.; Zhang, X.; Shi, W.; Chen, G.; Chen, W.; Lan, J.; Li, C.; Sun, W.; et al. Colorimetric detection of exosomal microRNA through switching the visible-light-induced oxidase mimic activity of acridone derivate. Biosens. Bioelectron. 2021, 173, 112834. [Google Scholar] [CrossRef] [PubMed]
- Agahi, M.; Rahaie, M. A novel DNA tweezers-based nanobiosensor for multiple detections of circulating exosomal microRNAs in breast cancer. Anal. Biochem. 2022, 651, 114697. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Wang, R.L.; Liu, L.; Cheng, L. A label-free colorimetric detection of microRNA via G-quadruplex-based signal quenching strategy. Anal. Chim. Acta 2019, 1079, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Mun, S.J.; Roh, Y.H.; Bong, K.W. Rapid colorimetric analysis of multiple microRNAs using encoded hydrogel microparticles. Analyst 2021, 146, 5508–5516. [Google Scholar] [CrossRef]
- Borghei, Y.S.; Hosseini, M.; Ganjali, M.R. Visual detection of miRNA using peroxidase-like catalytic activity of DNA-CuNCs and methylene blue as indicator. Clin. Chim. Acta Int. J. Clin. Chem. 2018, 483, 119–125. [Google Scholar] [CrossRef]
- Gong, S.; Wang, X.; Zhou, P.; Pan, W.; Li, N.; Tang, B. AND Logic-Gate-Based CRISPR/Cas12a Biosensing Platform for the Sensitive Colorimetric Detection of Dual miRNAs. Anal. Chem. 2022, 94, 15839–15846. [Google Scholar] [CrossRef]
- Ying, N.; Sun, T.; Chen, Z.; Song, G.; Qi, B.; Bu, S.; Sun, X.; Wan, J.; Li, Z. Colorimetric detection of microRNA based hybridization chain reaction for signal amplification and enzyme for visualization. Anal. Biochem. 2017, 528, 7–12. [Google Scholar] [CrossRef]
- Broto, M.; Kaminski, M.M.; Adrianus, C.; Kim, N.; Greensmith, R.; Dissanayake-Perera, S.; Schubert, A.J.; Tan, X.; Kim, H.; Dighe, A.S.; et al. Nanozyme-catalysed CRISPR assay for preamplification-free detection of non-coding RNAs. Nat. Nanotechnol. 2022, 17, 1120–1126. [Google Scholar] [CrossRef]
- La Spada, L. Metasurfaces for Advanced Sensing and Diagnostics. Sensors 2019, 19, 355. [Google Scholar] [CrossRef] [Green Version]
- Youssef, J.; Zhu, S.; Crunteanu, A.; Orlianges, J.C.; Ho, H.P.; Bachelot, R.; Zeng, S. Highly Sensitive Plasmonic Biosensors with Precise Phase Singularity Coupling on the Metastructures. Biosensors 2022, 12, 866. [Google Scholar] [CrossRef]
- La Spada, L.; Vegni, L. Electromagnetic Nanoparticles for Sensing and Medical Diagnostic Applications. Materials 2018, 11, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, M.; Shahbazzadeh, M.J.; La Spada, L.; Khajehzadeh, A. The Graphene Field Effect Transistor Modeling Based on an Optimized Ambipolar Virtual Source Model for DNA Detection. Appl. Sci-Basel 2021, 11, 8114. [Google Scholar] [CrossRef]
- Hucker, S.M.; Fehlmann, T.; Werno, C.; Weidele, K.; Luke, F.; Schlenska-Lange, A.; Klein, C.A.; Keller, A.; Kirsch, S. Single-cell microRNA sequencing method comparison and application to cell lines and circulating lung tumor cells. Nat. Commun. 2021, 12, 4316. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-Y.; Sun, M.-H.; Zhang, Q.; Li, P.-F.; Wang, K.; Li, X.-M. Advances in Point-of-Care Testing of microRNAs Based on Portable Instruments and Visual Detection. Biosensors 2023, 13, 747. https://doi.org/10.3390/bios13070747
Wang Z-Y, Sun M-H, Zhang Q, Li P-F, Wang K, Li X-M. Advances in Point-of-Care Testing of microRNAs Based on Portable Instruments and Visual Detection. Biosensors. 2023; 13(7):747. https://doi.org/10.3390/bios13070747
Chicago/Turabian StyleWang, Zhong-Yu, Ming-Hui Sun, Qun Zhang, Pei-Feng Li, Kun Wang, and Xin-Min Li. 2023. "Advances in Point-of-Care Testing of microRNAs Based on Portable Instruments and Visual Detection" Biosensors 13, no. 7: 747. https://doi.org/10.3390/bios13070747