Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
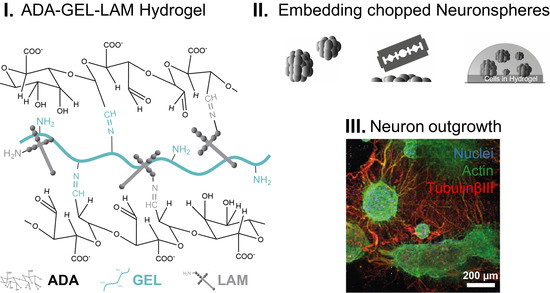
2.1. Materials Synthesis
2.2. Preparation of Hydrogels
2.3. Hydrogel Characterization
2.4. Cell Culture and Maintenance
2.5. Preparation of Sphere-Laden Hydrogels Subsection
2.6. Cytocompatibility Study
2.7. Immunocytochemistry
2.8. Statistical Analysis
3. Results
3.1. Material Characterization
3.2. Cytocompatibility of ADA-GEL-LAM Hydrogels
3.3. Neurospheres Differentiate into Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, A.M.; DeSimone, E.; Chwalek, K.; Kaplan, D.L. 3D in vitro modeling of the central nervous system. Prog. Neurobiol. 2015, 125, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, M. Reinventing clinical trials. Nat. Biotechnol. 2012, 30, 41–49. [Google Scholar] [CrossRef]
- Gribkoff, V.K.; Kaczmarek, L.K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 2017, 120, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, L.; Naciri, J.; Radio, N.; Tekur, S.; Clayton, D.; Apodaca, G.; Di Maio, R.; Zhi, Y.; Dimitrion, P.; Piazza, P.; et al. Generation of three-dimensional human neuronal cultures: Application to modeling CNS viral infections. Stem Cell Res. Ther. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centeno, E.G.Z.; Cimarosti, H.; Bithell, A. 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol. Neurodegener. 2018, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Elliott, N.T.; Yuan, F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J. Pharm. Sci. 2011, 100, 59–74. [Google Scholar] [CrossRef]
- Ko, K.R.; Frampton, J.P. Developments in 3D neural cell culture models: The future of neurotherapeutics testing? Expert Rev. Neurother. 2016, 16, 739–741. [Google Scholar] [CrossRef] [Green Version]
- Quadrato, G.; Brown, J.; Arlotta, P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med. 2016, 22, 1220–1228. [Google Scholar] [CrossRef]
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941. [Google Scholar] [CrossRef] [Green Version]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuFort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leipzig, N.D.; Shoichet, M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 2009, 30, 6867–6878. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate Modulus Directs Neural Stem Cell Behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [Green Version]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef]
- Flanagan, L.A.; Ju, Y.-E.; Marg, B.; Osterfield, M.; Janmey, P.A. Neurite branching on deformable substrates. Neuroreport 2002, 13, 2411–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zehnder, T.; Sarker, B.; Boccaccini, A.R.; Detsch, R. Evaluation of an alginate-gelatine crosslinked hydrogel for bioplotting. Biofabrication 2015, 7, 025001. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budday, S.; Sommer, G.; Birkl, C.; Langkammer, C.; Haybaeck, J.; Kohnert, J.; Bauer, M.; Paulsen, F.; Steinmann, P.; Kuhl, E.; et al. Mechanical characterization of human brain tissue. Acta Biomater. 2017, 48, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Schaller, E.; Steinmann, P.; Boccaccini, A.R.; Budday, S. Alginate-based hydrogels show the same complex mechanical behavior as brain tissue. J. Mech. Behav. Biomed. Mater. 2020, 111, 103979. [Google Scholar] [CrossRef]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, B.; Rompf, J.; Silva, R.; Lang, N.; Detsch, R.; Kaschta, J.; Fabry, B.; Boccaccini, A.R. Alginate-based hydrogels with improved adhesive properties for cell encapsulation. Int. J. Biol. Macromol. 2015, 78, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Hsueh, Y.; Tai, H.; Lin, F. Modifying alginate with early embryonic extracellular matrix, laminin, and hyaluronic acid for adipose tissue engineering. J. Biomed. Mater. Res. Part A 2015, 669–677. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.H.Y.; Tai, C.L.H.; Lin, F. Evaluation of a laminin-alginate biomaterial, adipocytes, and adipocyte-derived stem cells interaction in animal autologous fat grafting model using 7-Tesla magnetic resonance imaging. J. Mater. Sci. Mater. Med. 2017. [Google Scholar] [CrossRef]
- Distler, T.; Ruther, F.; Boccaccini, A.R.; Detsch, R. Development of 3D Biofabricated Cell Laden Hydrogel Vessels and a Low-Cost Desktop Printed Perfusion Chamber for In Vitro Vessel Maturation. Macromol. Biosci. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rottensteiner, U.; Sarker, B.; Heusinger, D.; Dafinova, D.; Rath, S.N.; Beier, J.P.; Kneser, U.; Horch, R.E.; Detsch, R.; Boccaccini, A.R.; et al. In vitro and in vivo biocompatibility of alginate dialdehyde/gelatin hydrogels with and without nanoscaled bioactive glass for bone tissue engineering applications. Materials 2014, 7, 1957–1974. [Google Scholar] [CrossRef]
- Reakasame, S.; Boccaccini, A.R. Oxidized Alginate-Based Hydrogels for Tissue Engineering Applications: A Review. Biomacromolecules 2018, 19, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Hofrichter, M.; Nimtz, L.; Tigges, J.; Kabiri, Y.; Schröter, F.; Royer-Pokora, B.; Hildebrandt, B.; Schmuck, M.; Epanchintsev, A.; Theiss, S.; et al. Comparative performance analysis of human iPSC-derived and primary neural progenitor cells (NPC) grown as neurospheres in vitro. Stem Cell Res. 2017, 25, 72–82. [Google Scholar] [CrossRef]
- Nimtz, L.; Hartmann, J.; Tigges, J.; Masjosthusmann, S.; Schmuck, M.; Keßel, E.; Theiss, S.; Köhrer, K.; Petzsch, P.; Adjaye, J.; et al. Characterization and application of electrically active neuronal networks established from human induced pluripotent stem cell-derived neural progenitor cells for neurotoxicity evaluation. Stem Cell Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, R.A.; Tholpady, S.S.; Foley, P.L.; Swami, N.; Ogle, R.C.; Botchwey, E.A. Alignment and composition of laminin-polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2012, 100, 406–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schackel, T.; Kumar, P.; Günther, M.; Liu, S.; Brunner, M.; Sandner, B.; Puttagunta, R.; Müller, R.; Weidner, N.; Blesch, A. Peptides and Astroglia Improve the Regenerative Capacity of Alginate Gels in the Injured Spinal Cord. Tissue Eng. Part A 2019, 25, 522–537. [Google Scholar] [CrossRef]
- Anderson, A.W.A.; Willenberg, A.R.; Bosak, A.J.; Willenberg, B.J.; Lambert, S. Use of a Capillary Alginate Gel (CapgelTM) to Study the Three-Dimensional Development of Sensory Nerves Reveals the Formation of A Rudimentary Perineurium. J. Neurosci. Methods 2018. [Google Scholar] [CrossRef]
- Yamada, Y.; Hozumi, K.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Biological activity of laminin peptide-conjugated alginate and chitosan matrices. Biopolymers 2010, 94, 711–720. [Google Scholar] [CrossRef]
- Hozumi, K.; Nomizu, M. Cell Adhesion Activity of Peptides Conjugated to Polysaccharides. Curr. Protoc. Cell Biol. 2018, 80. [Google Scholar] [CrossRef]
- Grigore, A.; Sarker, B.; Fabry, B.; Boccaccini, A.R.; Detsch, R. Behavior of Encapsulated MG-63 Cells in RGD and Gelatine-Modified Alginate Hydrogels. Tissue Eng. Part A 2014, 20, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Solistio, A.A.; Schneidereit, D.; Friedrich, O.; Detsch, R.; Boccaccini, A.R. 3D printed oxidized alginate-gelatin bioink provides guidance for C2C12 muscle precursor cell orientation and differentiation via shear stress during bioprinting. Biofabrication 2020. [Google Scholar] [CrossRef]
- Wu, P.; Tarasenko, Y.I.; Gu, Y.; Huang, L.Y.M.; Coggeshall, R.E.; Yu, Y. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat. Neurosci. 2002, 5, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.A.; Rebaza, L.M.; Derzic, S.; Schwartz, P.H.; Monuki, E.S. Regulation of human neural precursor cells by laminin and integrins. J. Neurosci. Res. 2006, 83, 845–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, X.; Josey, B.; James Chou, C.; Tan, Y.; Zhang, N.; Wen, X. Short Laminin Peptide for Improved Neural Stem Cell Growth. Stem Cells Transl. Med. 2014, 3, 662–670. [Google Scholar] [CrossRef]
- Tzu, J.; Marinkovich, M.P. Bridging structure with function: Structural, regulatory, and developmental role of laminins. Int. J. Biochem. Cell Biol. 2008, 40, 199–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsson, M. The role of laminin in attachment, growth, and differentiation of cultured cells: A brief review. Cytotechnology 1992, 9, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Georges-Labouesse, E.; Mark, M.; Messaddeq, N.; Gansmüller, A. Essential role of α6 integrins in cortical and retinal lamination. Curr. Biol. 1998, 8, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Campos, L.S.; Leone, D.P.; Relvas, J.B.; Brakebusch, C.; Fässler, R.; Suter, U.; Ffrench-Constant, C. β1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 2004, 131, 3433–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anlar, B.; Atilla, P.; Cakar, A.N.; Kose, M.F.; Beksac, M.S.; Dagdeviren, A.; Akcoren, Z. Expression of Adhesion and Extracellular Matrix Molecules in the Developing Human Brain. J. Child. Neurol. 2002, 17, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Heaton, M.B.; Swanson, D.J. The influence of laminin on the initial differentiation of cultured neural tube neurons. J. Neurosci. Res. 1988, 19, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective Differentiation of Neural Progenitor Cells by High-Epitope Density Nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, M.A.; He, X.; Wilkie, N.; Pollack, S.; Marshall, G.; Wafford, K.A.; Svendsen, C.N. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat. Biotechnol. 2001, 19, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, C.; Barenys, M.; Baumann, J.; Gassmann, K.; Casanellas, L.; Kauer, G.; Fritsche, E. Culture of human neurospheres in 3D scaffolds for developmental neurotoxicity testing. Toxicol. In Vitro 2018, 52, 106–115. [Google Scholar] [CrossRef]
- Zehnder, T.; Boccaccini, A.; Detsch, R. Biofabrication of a co-culture system in an osteoid-like hydrogel matrix. Biofabrication 2017, 9, 25016. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, L.; Willits, R.K. Neurite growth in PEG gels: Effect of mechanical stiffness and laminin concentration. J. Biomed. Mater. Res. Part. A 2011, 98A, 1–6. [Google Scholar] [CrossRef]
- Banerjee, A.; Arha, M.; Choudhary, S.S.; Ashton, R.R.; Bhatia, S.V.; Schaffer, D.S.; Kane, R. The Influence of Hydrogel Modulus on the Proliferation and Differentiation of Encapsulated Neural Stem Cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madl, C.M.; Lesavage, B.L.; Dewi, R.E.; Dinh, C.B.; Stowers, R.S.; Khariton, M.; Lampe, K.J.; Nguyen, D.; Chaudhuri, O.; Enejder, A.; et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017, 16, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N.; et al. Enzymatically crosslinked gelatin–laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Lopachin, R.M.; Gavin, T. Molecular Mechanisms of Aldehyde Toxicity: A Chemical Perspective. Chem. Res. Toxicol. 2014, 27, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Ishikawa, M.; Hayashi, T.; Yamada, Y.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Identification of cell adhesive sequences in the N-terminal region of the laminin α2 chain. J. Biol. Chem. 2012, 287, 25111–25122. [Google Scholar] [CrossRef] [Green Version]
- Kornev, V.A.; Grebenik, E.A.; Solovieva, A.B.; Dmitriev, R.I.; Timashev, P.S. Hydrogel-assisted neuroregeneration approaches towards brain injury therapy: A state-of-the-art review. Comput. Struct. Biotechnol. J. 2018, 16, 488–502. [Google Scholar] [CrossRef]
- Deister, C.; Aljabari, S.; Schmidt, C.E. Journal of Biomaterials Science, Effects of collagen 1, fibronectin, laminin and hyaluronic acid concentration in multi-component gels on neurite extension. J. Biomater. Sci. Polym. Ed. 2012, 37–41. [Google Scholar] [CrossRef]
- Barros, D.; Conde-sousa, E.; Gonçalves, A.M.; Han, W.M.; García, A.J.; Amaral, I.F.; Pêgo, A.P. Biomaterials Science as 3D neural stem cell culture systems. Biomater. Sci. 2019, 7, 5338–5349. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.T.; Mancino, R.J.; Bowles, R.D.; Brunger, J.M.; Tainter, D.M.; Chen, Y.; Richardson, W.J.; Guilak, F.; Setton, L.A. Biomaterials Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials 2013, 34, 7381–7388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addington, C.; Dharmaway, S.; Heffernan, J.; Sirianni, R.; Stabenfeldt, S. Hyaluronic acid-laminin hydrogels increase neural stem cell transplant retention and migratory response to SDF-1α. Matrix Biol. 2018, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Nakaji-hirabayashi, T.; Kato, K.; Iwata, H. In Vivo Study on the Survival of Neural Stem Cells Transplanted into the Rat Brain with a Collagen Hydrogel That Incorporates Laminin-Derived Polypeptides. Bioconjug. Chem. 2013, 24, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-kim, D.; Swindle-reilly, K.E.; Papke, J.B.; Kutosky, H.P.; Willits, R.K. The impact of laminin on 3D neurite extension in collagen gels. J. Neural Eng. 2012, 9, 046007. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Damodaran, G.; Nikolskaya, N.; Gorman, A.M.; Windebank, A.; Pandit, A. The effect of laminin peptide gradient in enzymatically cross-linked collagen scaffolds on neurite growth. J. Biomed. Mater. Res. Part A 2009, 92, 484–492. [Google Scholar] [CrossRef]
- Fujimori, C.; Kumai, J.; Nakamura, K.; Gu, Y. Biological activity of peptide-conjugated polyion complex matrices consisting of alginate and chitosan. Pept. Sci. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Zhao, J.; Chen, Y.M.; Zhang, P.; Zhang, Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.S.; Chen, Y.S.; Tai, H.C.; Mestak, O.; Chao, S.C.; Chen, Y.Y.; Shih, Y.; Lin, J.F.; Shieh, M.J.; Lin, F.H. Laminin-Alginate Beads as Preadipocyte Carriers to Enhance Adipogenesis in Vitro and in Vivo. Tissue Eng. Part A 2017, 23, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Llacua, A.; De Haan, B.J.; Smink, A.M.; Vos, P. De Extracellular matrix components supporting human islet function in alginate-based immunoprotective microcapsules for treatment of diabetes. J. Biomed. Mater. Res. Part A 2016, 1788–1796. [Google Scholar] [CrossRef]
- Marcinczyk, M.; Elmashhady, H.; Talovic, M.; Dunn, A. Laminin-111 enriched fibrin hydrogels for skeletal muscle regeneration. Biomaterials 2017, 141, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; ISBN 0900767782. [Google Scholar]
- Fritsche, E.; Haarmann-Stemmann, T.; Kapr, J.; Galanjuk, S.; Hartmann, J.; Mertens, P.R.; Kämpfer, A.A.M.; Schins, R.P.F.; Tigges, J.; Koch, K. Stem Cells for Next Level Toxicity Testing in the 21st Century. Small 2020, 2006252. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Distler, T.; Lauria, I.; Detsch, R.; Sauter, C.M.; Bendt, F.; Kapr, J.; Rütten, S.; Boccaccini, A.R.; Fritsche, E. Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels. Biomedicines 2021, 9, 261. https://doi.org/10.3390/biomedicines9030261
Distler T, Lauria I, Detsch R, Sauter CM, Bendt F, Kapr J, Rütten S, Boccaccini AR, Fritsche E. Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels. Biomedicines. 2021; 9(3):261. https://doi.org/10.3390/biomedicines9030261
Chicago/Turabian StyleDistler, Thomas, Ines Lauria, Rainer Detsch, Clemens M. Sauter, Farina Bendt, Julia Kapr, Stephan Rütten, Aldo R. Boccaccini, and Ellen Fritsche. 2021. "Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels" Biomedicines 9, no. 3: 261. https://doi.org/10.3390/biomedicines9030261