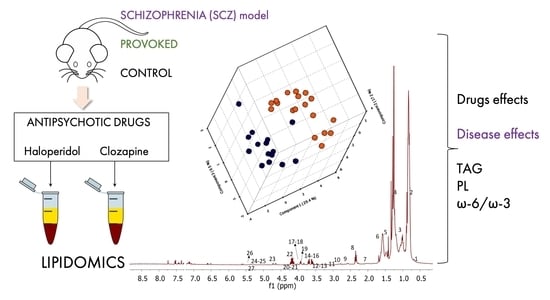
Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagents and Drugs
2.3. Drug-Naïve Animals
2.4. Treatment with Psychostimulants
2.5. Treatment with Antipsychotics
2.6. Extraction of Lipids from Serum Samples and NMR Analysis
2.7. Data Processing
3. Results
3.1. Identification of Lipids
3.2. Comparison of Lipids among Drug-Naïve NWR and SHR Animals, and NWR Receiving Psychostimulants
3.3. Influences of Antipsychotics HAL and CLZ on Lipids in SHR and NWR Animals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1 H NMR | Proton NMR |
| AMPH | Amphetamine |
| ANOVA | Analysis of variance |
| ChoGpl | Choline glycerophospholipids |
| Chol | Cholesterol |
| CLZ | Clozapine |
| FFA | Free fatty acids |
| HAL | Haloperidol |
| LOOCV | Leave-one-out cross-validation |
| LSDX | Lisdexamfetamine |
| MD | Mental disorder |
| NMR | Nuclear magnetic resonance |
| NWR | Normotensive Wistar rat |
| PCA | Principal component analysis |
| PL | Phospholipids |
| PLS-DA | Partial least squares discriminant analysis |
| PUFA | Polyunsaturated fatty acid |
| SCZ | Schizophrenia |
| SD | Standard deviation |
| SHR | Spontaneously hypertensive rat |
| UFA | Unsaturated fatty acids |
| VIP | Variable importance in projection |
References
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014, 39, 638–645. [Google Scholar]
- Murray, R.M.; Lappin, J.; Di Forti, M. Schizophrenia: From developmental deviance to dopamine dysregulation. Eur. Neuropsychopharmacol. 2008, 18, S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, B.; Nani, J.V.; Almeida, P.G.C.; Brietzke, E.; Lee, R.S.; Hayashi, M.A.F. Neuropeptides and oligopeptidases in schizophrenia. Neurosci. Biobehav. Rev. 2020, 108, 679–693. [Google Scholar] [CrossRef]
- Nucifora, F.C.; Woznica, E.; Lee, B.J.; Cascella, N.; Sawa, A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 2019, 131, 104257. [Google Scholar] [CrossRef] [PubMed]
- Ulivieri, M.; Wierońska, J.M.; Lionetto, L.; Martinello, K.; Cieslik, P.; Chocyk, A.; Curto, M.; Di Menna, L.; Iacovelli, L.; Traficante, A.; et al. The trace kynurenine, cinnabarinic acid, displays potent antipsychotic-like activity in mice and its levels are reduced in the prefrontal cortex of individuals affected by schizophrenia. Schizophr. Bull. 2020, 46, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Koola, M.M. Alpha7 nicotinic-N-methyl-D-aspartate hypothesis in the treatment of schizophrenia and beyond. Hum. Psychopharmacol. Clin. Exp. 2021, 36, 1–16. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Dyer, R.A.; Beasley, C.L. Evidence for altered cell membrane lipid composition in postmortem prefrontal white matter in bipolar disorder and schizophrenia. J. Psychiatr. Res. 2017, 95, 135–142. [Google Scholar] [CrossRef]
- Wood, P.L.; Unfried, G.; Whitehead, W.; Phillipps, A.; Wood, J.A. Dysfunctional plasmalogen dynamics in the plasma and platelets of patients with schizophrenia. Schizophr. Res. 2015, 161, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Tessier, C.; Sweers, K.; Frajerman, A.; Bergaoui, H.; Ferreri, F.; Delva, C.; Lapidus, N.; Lamaziere, A.; Roiser, J.P.; De Hert, M.; et al. Membrane lipidomics in schizophrenia patients: A correlational study with clinical and cognitive manifestations. Transl. Psychiatry 2016, 6, e906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nani, J.V.; Rodríguez, B.; Cruz, F.; Hayashi, M.A.F. Animal Models in Psychiatric Disorder Studies. In Animal Models in Medicine and Biology; Tvrdá, E., Yenisetti, S.C., Eds.; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Calzavara, M.B.; Medrano, W.A.; Levin, R.; Kameda, S.R.; Andersen, M.L.; Tufik, S.; Silva, R.H.; Frussa-Filho, R; Abilio, V.C. Neuroleptic drugs revert the contextual fear conditioning deficit presented by spontaneously hypertensive rats: A potential animal model of emotional context processing in schizophrenia? Schizophr. Bull. 2009, 35, 748–759. [Google Scholar]
- Levin, R.; Calzavara, M.B.; Santos, C.M.; Medrano, W.A.; Niigaki, S.T.; Abílio, V.C. Spontaneously hypertensive Rats (SHR) present deficits in prepulse inhibition of startle specifically reverted by clozapine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1748–1752. [Google Scholar] [CrossRef] [Green Version]
- Nani, J.V.; Yonamine, C.M.; Castro Musial, D.; Dal Mas, C.; Mari, J.J.; Hayashi, M.A.F. ACE activity in blood and brain axis in an animal model for schizophrenia: Demonstration of face validity related to ACE and predictive validity in response to antipsychotics. World J. Biol. Psychiatry 2019, 21, 1–11. [Google Scholar]
- Calzavara, M.B.; Levin, R.; Medrano, W.A.; Almeida, V.; Sampaio, A.P.F.; Barone, L.C.; Frussa-Filho, R.; Abílio, V.C. Effects of antipsychotics and amphetamine on social behaviors in spontaneously hypertensive rats. Behav. Brain Res. 2011, 225, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.; Machado, M.F.M.; Yonamine, C.M.; Sato, J.R.; Juliano, M.A.; Oliveira, V.; Bressan, R.A.; Hayashi, M.A.F. Plasma Ndel1 enzyme activity is reduced in patients with schizophrenia—a potential biomarker? J. Psychiatr. Res. 2013, 47, 657–663. [Google Scholar] [CrossRef]
- Gadelha, A.; Yonamine, C.M.; Ota, V.K.; Oliveira, V.; Sato, J.R.; Belangero, S.I.; Bressan, R.A.; Hayashi, M.A.F. ACE I/D genotype-related increase in ACE plasma activity is a better predictor for schizophrenia diagnosis than the genotype alone. Schizophr. Res. 2015, 164, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Dal Mas, C.; Nani, J.V.; Noto, C.; Yonamine, C.M.; da Cunha, G.R.; Mansur, R.B.; Ota, V.K.; Belangero, S.I.; Cordeiro, Q.; Kapczinski, F.; et al. Ndel1 oligopeptidase activity as a potential biomarker of early stages of schizophrenia. Schizophr. Res. 2019, 208, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Hayashi, M.A.F.; Barbosa, B.S.; Pontes, J.G.M.; Tasic, L.; Brietzke, E. Lipidomics, Biomarkers, and Schizophrenia: A Current Perspective. In Metabolomics: From Fundamentals to Clinical Applications. Advances in Experimental Medicine and Biology; Sussulini, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 965, pp. 265–290. [Google Scholar]
- Sethi, S.; Hayashi, M.A.F.; Sussulini, A.; Tasic, L.; Brietzke, E. Analytical approaches for lipidomics and its potential applications in neuropsychiatric disorders. World J. Biol. Psychiatry 2017, 18, 506–520. [Google Scholar] [CrossRef]
- Sethi, S.; Pedrini, M.; Rizzo, L.B.; Zeni-Graiff, M.; Mas, C.D.; Cassinelli, A.C.; Noto, M.N.; Asevedo, E.; Cordeiro, Q.; Pontes, J.G.M.; et al. 1H-NMR, 1H-NMR T2-edited, and 2D-NMR in bipolar disorder metabolic profiling. Int. J. Bipolar Disord. 2017, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tasic, L.; Pontes, J.G.M.; Carvalho, M.S.; Cruz, G.; Dal Mas, C.; Sethi, S.; Pedrini, M.; Rizzo, L.B.; ZeniGraiff, M.; Asevedo, E.; et al. Metabolomics and lipidomics analyses by 1H nuclear magnetic resonance of schizophrenia patient serum reveal potential peripheral biomarkers for diagnosis. Schizophr. Res. 2017, 185, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Tukiainen, T.; Tynkkynen, T.; Makinen, V.P.; Jylanki, P.; Kangas, A.; Hokkanen, J.; Vehtari, A.; Gröhn, O.; Hallikainen, M.; Soininen, H.; et al. A multi-metabolite analysis of serum by 1H NMR spectroscopy: Early systemic signs of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2008, 375, 356–361. [Google Scholar] [CrossRef]
- Mor, N.C.; Correia, B.S.B.; Val, A.L.; Tasic, L. A protocol for fish lipid analysis using nuclear magnetic resonance spectroscopy. J. Braz. Chem. Soc. 2020, 31, 662–672. [Google Scholar] [CrossRef]
- Li, J.; Vosegaard, T.; Guo, Z. Applications of nuclear magnetic resonance in lipid analyses: An emerging powerful tool for lipidomics studies. Prog. Lipid Res. 2017, 68, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.P.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. Quality of farmed and wild sea bass lipids studied by 1H NMR: Usefulness of this technique for differentiation on a qualitative and a quantitative basis. Food Chem. 2012, 135, 1583–1591. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; McEvoy, J.; Baillie, R.A.; Lee, D.; Yao, J.K.; Doraiswamy, P.M.; Krishnan, K.R.R. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol. Psychiatry 2007, 12, 934–945. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Yu, Z.; Xie, I.G.L.; Hartmann, A.M.; Prehn, C.; Adamski, J.; Kahn, R.; Li, Y.; Illig, T.; WangSattler, R.; et al. Schizophrenia shows a unique metabolomics signature in plasma. Transl. Psychiatry 2012, 2, e149. [Google Scholar] [CrossRef] [Green Version]
- Orešič, M.; Tang, J.; Seppänen-Laakso, T.; Mattila, I.; Saarni, S.E.; Saarni, S.I.; Lönnqvist, J.; Sysi-Aho, M.; Hyötyläinen, T.; Perälä, J.; et al. Metabolome in schizophrenia and other psychotic disorders: A general population-based study. Genome Med. 2011, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Orešič, M.; Seppänen-Laakso, T.; Sun, D.; Tang, J.; Therman, S.; Viehman, R.; Mustonen, U.; van Erp, T.G.; Hyötyläinen, T.; Thompson, P.; et al. Phospholipids and insulin resistance in psychosis: A lipidomics study of twin pairs discordant for schizophrenia. Genome Med. 2012, 4, 1–10. [Google Scholar] [CrossRef]
- McEvoy, J.; Baillie, R.A.; Zhu, H.; Buckley, P.; Keshavan, M.S.; Nasrallah, H.A.; Dougherty, G.G.; Yao, J.K.; Kaddurah-Daouk, R. Lipidomics reveals early metabolic changes in subjects with schizophrenia: Effects of atypical antipsychotics. PLoS ONE 2013, 8, e68717. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.K.; Stanley, J.A.; Reddy, R.D.; Keshavan, M.S.; Pettegrew, J.W. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol. Psychiatry 2002, 52, 823–830. [Google Scholar] [CrossRef]
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Paul, R.; Choudhury, A.; Kumar, S.; Giri, A.; Sandhir, R.; Borah, A. Cholesterol contributes to dopamine-neuronal loss in MPTP mouse model of Parkinson’s disease: Involvement of mitochondrial dysfunctions and oxidative stress. PLoS ONE 2017, 12, e0171285. [Google Scholar] [CrossRef] [Green Version]
- Ward, K.M.; Yeoman, L.; McHugh, C.; Kraal, A.Z.; Flowers, S.A.; Rothberg, A.E.; Karnovsky, A.; Das, A.K.; Ellingrod, V.L.; Stringer, K.A. Atypical antipsychotic exposure may not differentiate metabolic phenotypes of patients with schizophrenia. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 638–650. [Google Scholar] [CrossRef]
- Nandra, K.S.; Agius, M. The differences between typical and atypical antipsychotics: The effects on neurogenesis. Psychiatr. Danub. 2012, 24, S95–S99. [Google Scholar] [PubMed]
- Nasrallah, H.; Chen, A. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann. Clin. Psychiatry 2017, 29, 195–202. [Google Scholar] [PubMed]
- Paul, R.; Dutta, A.; Phukan, B.C.; Mazumder, M.K.; Justin-Thenmozhi, A.; Manivasagam, T.; Bhattacharya, P.; Borah, A. Accumulation of cholesterol and homocysteine in the nigrostriatal pathway of brain contributes to the dopaminergic neurodegeneration in mice. Neuroscience 2018, 388, 347–356. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, W.; Xu, N.; Xu, H.; Yu, C.; Liu, X.; Li, C. Polar metabolite of cholesterol induces rat cognitive dysfunctions. Neuroscience 2009, 164, 398–403. [Google Scholar] [CrossRef]
- Leoni, V.; Caccia, C. Study of cholesterol metabolism in Huntington’s disease. Biochem. Biophys. Res. Commun. 2014, 446, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Moutinho, M.; Nunes, M.J.; Rodrigues, E. Cholesterol 24-hydroxylase: Brain cholesterol metabolism and beyond. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M.; Kasimov, M.R.; Zefirov, A.L. Cholesterol in the pathogenesis of Alzheimer’s, Parkinson’s diseases and Autism: Link to synaptic dysfunction. Acta Nat. 2017, 9, 26–37. [Google Scholar] [CrossRef]
- Solberg, D.K.; Bentsen, H.; Refsum, H.; Andreassen, O.A. Lipid profiles in schizophrenia associated with clinical traits: A five year follow-up study. BMC Psychiatry 2016, 16, 299. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, S.R.; Kinnun, J.J.; Leng, X.; Williams, J.A.; Wassall, S.R. How polyunsaturated fatty acids modify molecular organization in membranes: Insight from NMR studies of model systems. Biochim. Biophys. Acta Biomembr. 2015, 1848, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Horrobin, D.F. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr. Res. 1998, 30, 193–208. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Mallinger, A.G.; Pettegrew, J.W.; Dippold, C. Erythrocyte membrane phospholipids in psychotic patients. Psychiatry Res. 1993, 49, 89–95. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Mukherjee, S.; Correnti, E.E.; Kelkar, H.S.; Wakade, C.G.; Costa, R.M.; Scheffer, R. Plasma membrane phospholipid and cholesterol distribution of skin fibroblasts from drug-naive patients at the onset of psychosis. Schizophr. Res. 1994, 13, 239–247. [Google Scholar] [CrossRef]
- Yang, J.; Chen, T.; Sun, L.; Zhao, Z.; Qi, X.; Zhou, K.; Cao, Y.; Wang, X.; Qiu, Y.; Su, M.; et al. Potential metabolite markers of schizophrenia. Mol. Psychiatry 2013, 18, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Levant, B.; Reichel, M.; Gulbins, E.; Kornhuber, J.; Müller, C.P. Lipids in psychiatric disorders and preventive medicine. Neurosci. Biobehav. Rev. 2017, 76, 336–362. [Google Scholar] [CrossRef] [Green Version]
- Satogami, K.; Takahashi, S.; Yamada, S.; Ukai, S.; Shinosaki, K. Omega-3 fatty acids related to cognitive impairment in patients with schizophrenia. Schizophr. Res. Cogn. 2017, 9, 8–12. [Google Scholar] [CrossRef]
- Rog, J.; Blazewicz, A.; Juchnowicz, D.; Ludwiczuk, A.; Stelmach, E.; Kozioł, M.; Karakula, M.; Nizinski, P.; Karakula-Juchnowicz, H. The role of GPR120 receptor in essential fatty acids metabolism in schizophrenia. Biomedicines 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Citrome, L. Lisdexamfetamine: Chemistry, pharmacodynamics, pharmacokinetics, and clinical efficacy, safety, and tolerability in the treatment of binge eating disorder. Expert Opin. Drug Metab. Toxicol. 2018, 14, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Nani, J.V.; Lee, R.S.; Yonamine, C.; Dal Mas, C.; Sant’Anna, O.; Juliano, M.A.; Gadelha, A.; Mari, J.J.; Hayashi, M.A.F. Evaluation of NDEL1 oligopeptidase activity in blood and brain in an animal model of schizophrenia: Effects of psychostimulants and antipsychotics. Sci. Rep. 2020, 10, 18513. [Google Scholar] [CrossRef] [PubMed]
- Hejr, H.; Ghareghani, M.; Zibara, K.; Ghafari, M.; Sadri, F.; Salehpour, Z.; Hamedi, A.; Negintaji, K.; Azari, H.; Ghanbari, A. The ratio of 1/3 linoleic acid to alpha linolenic acid is optimal for oligodendrogenesis of embryonic neural stem cells. Neurosci. Lett. 2017, 651, 216–225. [Google Scholar] [CrossRef]
- Bourre, J.M.; Dumont, O. Dietary oleic acid not used during brain development and in adult in rat, in contrast with sciatic nerve. Neurosci. Lett. 2003, 336, 180–184. [Google Scholar] [CrossRef]
- Bourre, J.M. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J. Nutr. Health Aging 2004, 8, 163–174. [Google Scholar] [PubMed]
- Berman, S.M.; Kuczenski, R.; McCracken, J.T.; London, E.D. Potential adverse effects of amphetamine treatment on brain and behavior: A review. Mol. Psychiatry 2009, 14, 123–142. [Google Scholar] [CrossRef]
- Thomas, D.M.; Francescutti-Verbeem, D.M.; Kuhn, D.M. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J. Neurochem. 2008, 105, 605–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.W.; Seo, M.K.; McIntyre, R.S.; Mansur, R.B.; Lee, Y.; Lee, J.-H.; Park, S.-C.; Huh, L.; Lee, J.G. Effects of olanzapine and haloperidol on mTORC1 signaling dendritic outgrowth, and synaptic proteins in rat primary hippocampal neurons under toxic conditions. Neurosci. Lett. 2018, 686, 59–66. [Google Scholar] [CrossRef]
- Tessier, C.; Nuss, P.; Staneva, G.; Wolf, C. Modification of membrane heterogeneity by antipsychotic drugs: An X-ray diffraction comparative study. J. Colloid Interface Sci. 2008, 320, 469–479. [Google Scholar] [CrossRef]


| Peak | Chemical Shift (ppm) | Assignment |
|---|---|---|
| 1 | 0.58–0.70 | Terminal methyl group in cholesterol -CH3 |
| 2 | 0.75–1.00 | -CH3 protons of saturated, oleic and linoleic acyls (omega-6) |
| 3 | 0.93–1.02 | -CH3 protons of linolenyl chain (omega-3) |
| 4 | 1.20–1.50 | Methylene protons of aliphatic chains -(CH2)n |
| 5 | 1.50–1.75 | β–methylene protons of the carbonyl –OC(O)-CH2-CH2- |
| 6 | 1.95–2.10 | Methylene protons in the α-position of double bonds –CH2-CH=CH- |
| 7 | 2.20–2.50 | Methylene protons in the carbonyl α-position –OC(O)-CH2- |
| 8 | 2.70–2.84 | CH2-bis-allyllic protons of polyunsaturated fatty acid (PUFA) chains |
| 9 | 2.80–2.90 a | Divinyl methylene protons =HC-CH2-CH= of omega-6 including linoleyl chain |
| 10 | 2.79 b | Divinyl methylene protons =HC-CH2-CH= of omega-3 including linolenyl chain |
| 11 | 3.10–3.20 | Methylene protons α to the heteroatom –CH2-OH |
| 12 | 3.20–3.40 | Methyl protons of charged nitrogen –+N(CH3)3 |
| 13 | 3.40–3.60 | Heteroatom proton –OH |
| 14 | 3.44–3.59 | CH of cholesterol relative to the C-3 proton |
| 15 | 3.50–3.85 | Methylene protons α to a charged nitrogen CH2-N+(CH3)3 |
| 16 | 3.65–3.75 | Hexoses protons on α-carbon to the heteroatom |
| 17 | 3.88 | Methine proton at C-4 of galactose |
| 18 | 4.00–4.30 | Protons on α-carbon to the heteroatom |
| 19 | 4.10–4.40 | Protons on α-carbon to the heteroatom (OH) and β to the amine -O-CH2-CH2-N+(CH3)3 |
| 20 | 3.90–4.40 | Methylene protons α to the heteroatom in phosphorus CH2-O-P |
| 21 | 4.10–4.30 c | Sn-1 and Sn-3 protons of glycerol -CH2-OC(O)R |
| 22 | 5.00 | Anomeric carbon protons of galactose |
| 23 | 5.20–5.40 | Amine protons –HN(CH3)2 |
| 24 | 5.25–5.50 | Sn-2 protons of glycerol > CH-O-C(O)R |
| 25 | 5.27–5.38 | Protons of double bonds with conformation Z-CH=HC- |
| Omega 3 (%) | Omega 6 (%) | Omega 6/3 | ||
|---|---|---|---|---|
| Groups | NWR | 50.16 ± 9.04 | 25.74 ± 5.18 | 0.52 ± 0.05 |
| SHR | 26.37 ± 5.39 | 7.43 ± 1.06 | 0.29 ± 0.10 | |
| NWR-AMPH * | 5.41 ± 1.56 | 22.28 ± 7.68 | 4.12 ± 1.30 | |
| NWR-AMPH ** | 6.03 ± 2.52 | 25.86 ± 3.10 | 4.66 ± 1.25 | |
| NRW-LSDX * | 10.10 ± 3.75 | 43.50 ± 17.40 | 4.33 ± 0.81 | |
| NRW-LSDX ** | 10.13 ± 2.55 | 33.65 ± 6.05 | 3.49 ± 1.05 | |
| p-Values | NWR × SHR | 0.0025 (t = 5.24) | 0.0002 (t = 6.85) | 0.0042 (t = 4.16) |
| NWR × NWR-AMPH * | <0.0001 (t = 10.90) | 0.5400 (t = 0.83) | 0.0003 (t = 6.15) | |
| NWR × NWR-AMPH ** | <0.0001 (t = 10.51) | 0.9992 (t = 0.042) | < 0.0001 (t = 7.41) | |
| NWR × NWR-AMPH * + NWR-AMPH ** | <0.0001 (F (2, 14) = 109.0) | 0.5403 (F (2, 14) = 0.64) | < 0.0001 (F (2, 14) = 23.26) | |
| NWR × NWR-LSDX* | <0.0001 (t = 9.14) | 0.0467 (t = 2.57) | < 0.0001 (t = 10.42) | |
| NWR × NWR-LSDX ** | <0.0001 (t = 9.52) | 0.4440 (t = 2.31) | < 0.0001 (t = 6.28) | |
| NWR × NWR-LSDX * + NWR-LSDX ** | <0.0001 (F (2, 14) = 78.27) | 0.0749 (F (2, 14) = 3.24) | < 0.0001 (F (2, 14) = 33.74) | |
| SHR × NWR-AMPH * + NWR-AMPH ** | <0.0001 (F (2, 14) = 54.64) | 0.0005 (F (2, 14) = 16.30) | 0.0002 (F (2, 14) = 20.37) | |
| SHR × NWR-LSDX * + NWR-LSDX ** | <0.0001 (F (2, 14) = 24.42) | 0.0016 (F (2, 14) = 12.18) | < 0.0001 (F (2, 14) = 29.97) |
| Omega-3 (%) | Omega-6 (%) | Omega 6/3 | ||
|---|---|---|---|---|
| Groups | NWR | 50.16 ± 9.04 | 25.74 ± 5.18 | 0.52 ± 0.05 |
| SHR | 26.37 ± 5.39 | 7.43 ± 1.06 | 0.29 ± 0.10 | |
| NWR-HAL | 0.75 ± 0.26 | 2.15 ± 0.54 | 3.02 ± 0.71 | |
| SHR-HAL | 0.92 ± 0.09 | 2.96 ± 0.77 | 3.16 ± 0.62 | |
| NWR-CLZ | 0.90 ± 0.44 | 2.69 ± 1.48 | 2.87 ± 1.42 | |
| SHR-CLZ | 1.00 ± 0.57 | 1.07 ± 0.50 | 2.60 ± 2.08 | |
| p-Values | NWR × SHR | 0.0019 (t = 5.24) | 0.0002 (t = 7.83) | 0.0042 (t = 3.74) |
| NWR × NWR-HAL | < 0.0001 (t = 14.05) | <0.0001 (t = 11.48) | 0.0037 (t = 6.89) | |
| NWR × NWR-CLZ | < 0.0001 (t = 14.03) | <0.0001 (t = 10.71) | 0.0163 (t = 3.31) | |
| NWR × NWR-HAL + NWR-CLZ | <0.0001 (F (2, 14) =195.4) | <0.0001 (F (2, 14) = 114.4) | 0.0708 (F (2, 14) = 3.41) | |
| SHR × SHR-HAL | <0.0001 (t = 10.73) | 0.0002 (t = 7.27) | 0.0110 (t = 8.92) | |
| SHR × SHR-CLZ | <0.0001 (t = 10.68) | <0.0001 (t = 10.75) | 0.0456 (t = 2.66) | |
| SHR × SHR-HAL + SHR-CLZ | <0.0001 (F (2, 14) = 114.6) | <0.0001 (F (2, 14) = 64.81) | 0.0143 (F (2, 14) = 6.69) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, B.S.B.; Nani, J.V.; Waladares Ricardo, R.; Stanisic, D.; Costa, T.B.B.C.; Hayashi, M.A.F.; Tasic, L. Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia. Biomedicines 2021, 9, 235. https://doi.org/10.3390/biomedicines9030235
Correia BSB, Nani JV, Waladares Ricardo R, Stanisic D, Costa TBBC, Hayashi MAF, Tasic L. Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia. Biomedicines. 2021; 9(3):235. https://doi.org/10.3390/biomedicines9030235
Chicago/Turabian StyleCorreia, Banny Silva Barbosa, João Victor Nani, Raniery Waladares Ricardo, Danijela Stanisic, Tássia Brena Barroso Carneiro Costa, Mirian A. F. Hayashi, and Ljubica Tasic. 2021. "Effects of Psychostimulants and Antipsychotics on Serum Lipids in an Animal Model for Schizophrenia" Biomedicines 9, no. 3: 235. https://doi.org/10.3390/biomedicines9030235