Neuroprotection: Targeting Multiple Pathways by Naturally Occurring Phytochemicals
Abstract
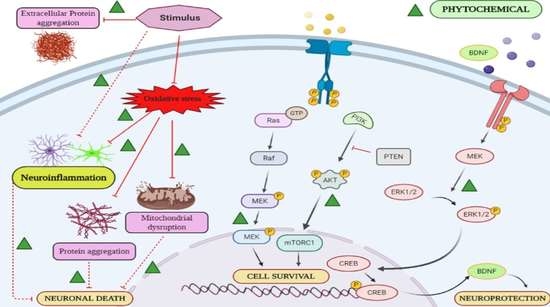
:1. Introduction
2. Methodology
3. Multifunctional Phytochemicals as Novel Therapeutic Agents for Neurodegenerative Disorders
3.1. Alzheimer’s Disease and Related Dementias
3.2. Parkinson’s Disease and Parkinsonism
3.3. Amyotrophic Lateral Sclerosis
3.4. Huntington’s Disease
3.5. Vascular Cognitive Impairment
3.6. Prion Disease
3.7. Frontotemporal Dementia (FTD)
3.8. Spinocerebellar Ataxia (SCA)
3.9. Spinal Muscular Atrophy (SMA)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wynford-Thomas, R.; Robertson, N.P. The economic burden of chronic neurological disease. J. Neurol. 2017, 264, 2345–2347. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Pan, W. The Treatment Strategies for Neurodegenerative Diseases by Integrative Medicine. Integr. Med. Int. 2015, 1, 223–225. [Google Scholar] [CrossRef]
- Mizuno, Y. Recent Research Progress in and Future Perspective on Treatment of Parkinson’s Disease. Integr. Med. Int. 2014, 1, 67–79. [Google Scholar] [CrossRef]
- Shukla, A.W.; Shuster, J.J.; Chung, J.W.; Vaillancourt, D.E.; Patten, C.; Ostrem, J.; Okun, M.S. Repetitive Transcranial Magnetic Stimulation (rTMS) Therapy in Parkinson Disease: A Meta-Analysis. PM&R 2015, 8, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Bordet, R.; Ihl, R.; Korczyn, A.D.; Lanza, G.; Jansa, J.; Hoerr, R.; Guekht, A. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: A consensus report. BMC Med. 2017, 15, 107. [Google Scholar] [CrossRef]
- Fisicaro, F.; Lanza, G.; Grasso, A.A.; Pennisi, G.; Bella, R.; Paulus, W.; Pennisi, M. Repetitive transcranial magnetic stimulation in stroke rehabilitation: Review of the current evidence and pitfalls. Ther. Adv. Neurol. Disord. 2019, 12. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yan, T.; Chu, J.M.-T.; Chen, Y.; Dunnett, S.; Ho, Y.-S.; Wong, G.T.C.; Chang, R.C.C. The beneficial effects of physical exercise in the brain and related pathophysiological mechanisms in neurodegenerative diseases. Lab. Investig. 2019, 99, 943–957. [Google Scholar] [CrossRef]
- Lanza, G.; Pino, M.; Fisicaro, F.; Vagli, C.; Cantone, M.; Pennisi, M.; Bella, R.; Bellomo, M. Motor activity and Becker’s muscular dystrophy: Lights and shadows. Physician Sportsmed. 2019, 48, 151–160. [Google Scholar] [CrossRef]
- Lanza, G.; Casabona, J.A.; Bellomo, M.; Cantone, M.; Fisicaro, F.; Bella, R.; Pennisi, G.; Bramanti, P.; Pennisi, M.; Bramanti, A. Update on intensive motor training in spinocerebellar ataxia: Time to move a step forward? J. Int. Med Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Lanza, G.; Centonze, S.S.; Destro, G.; Vella, V.; Bellomo, M.; Pennisi, M.; Bella, R.; Ciavardelli, D. Shiatsu as an adjuvant therapy for depression in patients with Alzheimer’s disease: A pilot study. Complement. Ther. Med. 2018, 38, 74–78. [Google Scholar] [CrossRef]
- Prasansuklab, A.; Brimson, J.M.; Tencomnao, T. Potential Thai medicinal plants for neurodegenerative diseases: A review focusing on the anti-glutamate toxicity effect. J. Tradit. Complement. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Lanza, G.; Cantone, M.; D’Amico, E.; Fisicaro, F.; Puglisi, V.; Vinciguerra, L.; Bella, R.; Vicari, E.; Malaguarnera, G. Acetyl-L-Carnitine in Dementia and Other Cognitive Disorders: A Critical Update. Nutrients 2020, 12, 1389. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Cree, I.A. High-Throughput Screening of Natural Products for Cancer Therapy. Planta Medica 2010, 76, 1080–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.L.; Clark, R.L.; Mackay, S.P.; Johnston, B.F. Current strategies for drug discovery through natural products. Expert Opin. Drug Discov. 2010, 5, 559–568. [Google Scholar] [CrossRef]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals That Regulate Neurodegenerative Disease by Targeting Neurotrophins: A Comprehensive Review. BioMed Res. Int. 2015, 2015, 1–22. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Farkhondeh, T.; Samarghandian, S.; Pourbagher-Shahri, A.M.; Sedaghat, M. The impact of curcumin and its modified formulations on Alzheimer’s disease. J. Cell. Physiol. 2019, 234, 16953–16965. [Google Scholar] [CrossRef]
- Macdonald, I.R.; Rockwood, K.; Martin, E.; Darvesh, S. Cholinesterase Inhibition in Alzheimer’s Disease: Is Specificity the Answer? J. Alzheimer’s Dis. 2014, 42, 379–384. [Google Scholar] [CrossRef]
- Webber, K.M.; Raina, A.K.; Marlatt, M.W.; Zhu, X.; Prat, M.I.; Morelli, L.; Casadesus, G.; Perry, G.; Smith, M.A. The cell cycle in Alzheimer disease: A unique target for neuropharmacology. Mech. Ageing Dev. 2005, 126, 1019–1025. [Google Scholar] [CrossRef]
- Ganguli, M.; Chandra, V.; Kamboh, M.I.; Johnston, J.M.; Dodge, H.H.; Thelma, B.K.; Juyal, R.C.; Pandav, R.; Belle, S.H.; DeKosky, S.T. Apolipoprotein E Polymorphism and Alzheimer Disease. Arch. Neurol. 2000, 57, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Oghabian, Z.; Mehrpour, O. Treatment of Aluminium Phosphide Poisoning with a Combination of Intravenous Glucagon, Digoxin and Antioxidant Agents. Sultan Qaboos Univ. Med J. 2016, 16, e352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khani, A.H.; Sahragard, A.; Namdari, A.; Zarshenas, M.M. Botanical Sources for Alzheimer’s: A Review on Reports from Traditional Persian Medicine. Am. J. Alzheimer’s Dis. Other Dement. 2017, 32, 429–437. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Roselin, P.; Singh, K.K.; John, Z.; Saxena, S.N. Postharvest processing and benefits of black pepper, coriander, cinnamon, fenugreek, and turmeric spices. Crit. Rev. Food Sci. Nutr. 2016, 56, 1585–1607. [Google Scholar] [CrossRef]
- Lin, J.-K. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. In Molecular Targets of Curcumin; Aggarwal, B.B., Surh, Y.J., Shishodia, S., Eds.; Springer: Boston, MA, USA, 2007; Volume 595, pp. 227–243. [Google Scholar] [CrossRef]
- Liu, Z.; Li, T.; Yang, D.; Smith, W.W. Curcumin protects against rotenone-induced neurotoxicity in cell and drosophila models of Parkinson’s disease. Adv. Park. Dis. 2013, 2, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.-Y.; Zhang, L.; Li, H.; Liu, T.-L.; Lai, J.-C.; Wu, Z.-B.; Qin, J. Protective effects of curcumin on acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Pharmacol. Rep. 2018, 70, 1040–1046. [Google Scholar] [CrossRef]
- Maan, G.; Sikdar, B.; Kumar, A.; Shukla, R.; Mishra, A. Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top. Med. Chem. 2020, 20, 1169–1194. [Google Scholar] [CrossRef]
- Sundaram, J.R.; Poore, C.P.; Bin Sulaimee, N.H.; Pareek, T.; Cheong, W.F.; Wenk, M.R.; Pant, H.C.; Frautschy, S.A.; Low, C.-M.; Kesavapany, S. Curcumin Ameliorates Neuroinflammation, Neurodegeneration, and Memory Deficits in p25 Transgenic Mouse Model that Bears Hallmarks of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 1429–1442. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.-K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Frost, S.; Kanagasingam, Y.; Macaulay, L.; Koronyo-Hamaoui, M.; Koronyo, Y.; Biggs, D.; Verdooner, S.; Black, K.; Taddei, K.; Shah, T.; et al. O3-13-01: Retinal amyloid fluorescence imaging predicts cerebral amyloid burden and alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 234–235. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.-L.; Wang, Y.-R.; Fa, X.-Z. Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res. 2013, 1492, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, H.; Wagner, A.E.; Saadatmandi, C.B.; Kruse, H.-P.; Kulling, S.; Rimbach, G. Effect of dietary genistein on Phase II and antioxidant enzymes in rat liver. Cancer Genom. Proteom. 2009, 6, 85–92. [Google Scholar]
- Marchenko, N.D.; Zaika, A.; Moll, U.M. Death signal-induced localization of p53 protein to mitochondria a potential role in apoptotic signaling. J. Biol. Chem. 2000, 275, 16202–16212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jimenez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mahapatra, P.; Kumari, P.; Kushwaha, P.P.; Singh, P.; Kumar, S. Phytochemical as Hope for the Treatment of Hepatic and Neuronal Disorders. Phytochemistry 2018, 2, 289–314. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases; Academic Press: Salt Lake, UT, USA, 2017; p. 484. [Google Scholar] [CrossRef]
- Ong, W.-Y.; Farooqui, T.; Ho, C.F.-Y.; Ng, Y.-K.; Farooqui, A.A. Use of Phytochemicals against Neuroinflammation. Neuroprotective Effects Phytochem. Neurol. Disord. 2017, 1–41. [Google Scholar] [CrossRef]
- Lee, T.-H.; Jung, M.; Bang, M.-H.; Chung, D.K.; Kim, J.-Y. Inhibitory effects of a spinasterol glycoside on lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines via down-regulating MAP kinase pathways and NF-κB activation in RAW264.7 macrophage cells. Int. Immunopharmacol 2012, 13, 264–270. [Google Scholar] [CrossRef]
- Liao, W.; Jin, G.; Zhao, M.; Yang, H. The Effect of Genistein on the Content and Activity of α- and β-Secretase and Protein Kinase C in Aβ-Injured Hippocampal Neurons. Basic Clin. Pharmacol. Toxicol. 2012, 112, 182–185. [Google Scholar] [CrossRef]
- Ye, S.; Wang, T.T.; Cai, B.; Wang, Y.; Li, J.; Zhan, J.X.; Shen, G.M. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer’s disease model rats. Neural Regen. Res. 2017, 12, 1479–1484. [Google Scholar] [CrossRef]
- Kuo, L.-C.; Song, Y.-Q.; Yao, C.-A.; Cheng, I.H.; Chien, C.-T.; Lee, G.-C.; Yang, W.-C.; Lin, Y. Ginkgolide A. Prevents the Amyloid-β-Induced Depolarization of Cortical Neurons. J. Agric. Food Chem. 2018, 67, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Tchantchou, F.; Xu, Y.; Wu, Y.; Christen, Y.; Luo, Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007, 21, 2400–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Tabaa, M.M.; Sokkar, S.S.; Ramadan, E.S.; El Salam, I.Z.A.; Zaid, A.M. Neuroprotective role of Ginkgo biloba against cognitive deficits associated with Bisphenol A exposure: An animal model study. Neurochem. Int. 2017, 108, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, X.-X.; He, G.-R.; Hu, J.-J.; Mu, X.; Tian, S.; Du, G.-H. Luteolin promotes long-term potentiation and improves cognitive functions in chronic cerebral hypoperfused rats. Eur. J. Pharmacol. 2010, 627, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, V.; Baier, C.J.; Murray, M.; Estévez-Braun, A.; Murray, A.P. Neuroprotective effects of Flaveria bidentis and Lippia salsa extracts on SH-SY5Y cells. S. Afr. J. Bot. 2018, 119, 318–324. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Cheng, H.; Che, Z. Ameliorating effect of luteolin on memory impairment in an Alzheimer’s disease model. Mol. Med. Rep. 2016, 13, 4215–4220. [Google Scholar] [CrossRef] [Green Version]
- Pappolla, M.A.; Sos, M.; Omar, R.A.; Bick, R.J.; Hickson-Bick, D.L.M.; Reiter, R.J.; Efthimiopoulos, S.; Robakis, N.K. Melatonin Prevents Death of Neuroblastoma Cells Exposed to the Alzheimer Amyloid Peptide. J. Neurosci. 1997, 17, 1683–1690. [Google Scholar] [CrossRef]
- Nie, L.; Wei, G.; Peng, S.; Qu, Z.; Yang, Y.; Yang, Q.; Huang, X.; Liu, J.; Zhuang, Z.; Yang, X. Melatonin ameliorates anxiety and depression-like behaviors and modulates proteomic changes in triple transgenic mice of Alzheimer’s disease. BioFactors 2017, 43, 593–611. [Google Scholar] [CrossRef]
- Singer, C.; Tractenberg, R.E.; Kaye, J.A.; Schafer, K.; Gamst, A.; Grundman, M.; Thomas, R.G.; Thal, L.J. A Multicenter, Placebo-controlled Trial of Melatonin for Sleep Disturbance in Alzheimer’s Disease. Sleep 2003, 26, 893–901. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, Z.; Zhang, Z.; Liu, G.; Wang, Y.; Ren, Y.; Wu, X.; Geng, F. Protective Role of Naringenin Against Aβ25-35-Caused Damage via ER and PI3K/Akt-Mediated Pathways. Cell. Mol. Neurobiol. 2017, 38, 549–557. [Google Scholar] [CrossRef]
- Haider, S.; Liaquat, L.; Ahmad, S.; Batool, Z.; Ali Siddiqui, R.; Tabassum, S.; Shahzad, S.; Rafiq, S.; Naz, N. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS ONE 2020, 15, e0227631. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, Z.; Wang, W.; Wei, M.; Kou, D.; Li, T.; Yang, Z.; Guo, H.; Le, W.; Li, S. Piperine attenuates cognitive impairment in an experimental mouse model of sporadic Alzheimer’s disease. J. Nutr. Biochem. 2019, 70, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, S.; Li, J.; Sun, Y.; Hasimu, H.; Liu, R.; Zhang, T. Quercetin protects human brain microvascular endothelial cells from fibrillar β-amyloid1–40-induced toxicity. Acta Pharm. Sin. B 2015, 5, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabogal-Guáqueta, A.M.; Manco, J.I.M.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Jiang, T.; Li, W.; Gao, N.; Zhang, T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol. Lett. 2018, 282, 100–108. [Google Scholar] [CrossRef]
- Porquet, D.; Griñán-Ferré, C.; Ferrer, I.; Camins, A.; Sanfeliu, C.; Del Valle, J.; Pallàs, M. Neuroprotective Role of Trans-Resveratrol in a Murine Model of Familial Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 42, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. NY Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef]
- Khan, A.; Vaibhav, K.; Javed, H.; Khan, M.M.; Tabassum, R.; Ahmed, E.; Srivastava, P.; Khuwaja, G.; Islam, F.; Siddiqui, M.S.; et al. Attenuation of Aβ-induced neurotoxicity by thymoquinone via inhibition of mitochondrial dysfunction and oxidative stress. Mol. Cell. Biochem. 2012, 369, 55–65. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar] [CrossRef]
- Tsai, S.-J.; Chiu, C.P.; Yang, H.T.; Yin, M.C. s-Allyl cysteine, s-ethyl cysteine, and s-propyl cysteine alleviate β-amyloid, glycative, and oxidative injury in brain of mice treated by D-galactose. J. Agric. Food Chem. 2011, 59, 6319–6326. [Google Scholar] [CrossRef]
- Khan, A.; Vaibhav, K.; Javed, H.; Tabassum, R.; Ahmed, E.; Khan, M.M.; Khan, M.B.; Shrivastava, P.; Islam, F.; Siddiqui, M.S.; et al. 1,8-Cineole (Eucalyptol) Mitigates Inflammation in Amyloid Beta Toxicated PC12 Cells: Relevance to Alzheimer’s Disease. Neurochem. Res. 2013, 39, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Li, X.-Y.; Ji, H.-F. Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl. Microbiol. Biotechnol. 2019, 103, 7141–7149. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, B.; Cui, L.; Zhou, B.; Liu, W.; Xu, F.; Hayashi, T.; Hattori, S.; Ushiki-Kaku, Y.; Tashiro, S.-I.; et al. Silibinin ameliorates anxiety/depression-like behaviors in amyloid β-treated rats by upregulating BDNF/TrkB pathway and attenuating autophagy in hippocampus. Physiol. Behav. 2017, 179, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-Enhancing Effect of Quercetin in a Rat Model of Parkinson’s Disease Induced by 6-Hydroxydopamine. Evid. Based Complement. Altern. Med. 2011, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, Z.-L.; Wu, H.-C.; Hu, Q.-L.; Zhang, S.-J.; Wang, Y.-M.; Jin, Z.-K.; Lv, L.-F.; Wu, H.-L.; Cheng, O.-M. Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural Regen. Res. 2018, 13, 1375–1383. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Naz, F.; Jyoti, S.; Ali, F. Rahul Effect of Genistein on the Transgenic Drosophila Model of Parkinson’s Disease. J. Diet. Suppl. 2018, 16, 550–563. [Google Scholar] [CrossRef]
- Jung, U.J.; Kim, S.R. Effects of naringin, a flavanone glycoside in grapefruits and citrus fruits, on the nigrostriatal dopaminergic projection in the adult brain. Neural Regen. Res. 2014, 9, 1514–1517. [Google Scholar] [CrossRef]
- Sang, Q.; Liu, X.; Wang, L.; Qi, L.; Sun, W.; Wang, W.; Sun, Y.; Zhang, H. Curcumin Protects an SH-SY5Y Cell Model of Parkinson’s Disease Against Toxic Injury by Regulating HSP90. Cell. Physiol. Biochem. 2018, 51, 681–691. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2017, 26, 349–360. [Google Scholar] [CrossRef]
- Chung, W.-G.; Miranda, C.L.; Maier, C.S. Epigallocatechin gallate (EGCG) potentiates the cytotoxicity of rotenone in neuroblastoma SH-SY5Y cells. Brain Res. 2007, 1176, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Zhu, M.; Liang, Z. (-)-Epigallocatechin-3-gallate modulates peripheral immunity in the MPTP-induced mouse model of Parkinson’s disease. Mol. Med. Rep. 2018, 17, 4883–4888. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Fan, W.; Wang, H.; Bao, L.; Li, G.; Li, T.; Song, S.; Li, H.; Hao, J.; Sun, J. Resveratrol Protects PC12 Cell against 6-OHDA Damage via CXCR4 Signaling Pathway. Evid. Based Complement. Altern. Med. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, D.; Sui, R.; Zhang, Z. Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J. Cell. Biochem. 2019, 120, 4942–4951. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Protective effects of quercetin glycosides, rutin, and isoquercetrin against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in rat pheochromocytoma (PC-12) cells. Int. J. Immunopathol. Pharmacol. 2015, 29, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.M.; Raza, S.S.; Javed, H.; Ahmad, A.; Khan, A.; Islam, F.; Safhi, M.M.; Islam, F. Rutin Protects Dopaminergic Neurons from Oxidative Stress in an Animal Model of Parkinson’s Disease. Neurotox. Res. 2011, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.S.; Goes, A.T.; Boeira, S.P.; Prigol, M.; Jesse, C.R. Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition 2014, 30, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, P.; Vaibhav, K.; Tabassum, R.; Khan, A.; Ishrat, T.; Khan, M.M.; Ahmad, A.; Islam, F.; Safhi, M.M.; Islam, F. Anti-apoptotic and Anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson’s Rat model. J. Nutr. Biochem. 2013, 24, 680–687. [Google Scholar] [CrossRef]
- Jiang, F.; DeSilva, S.; Turnbull, J. Beneficial effect of ginseng root in SOD-1 (G93A) transgenic mice. J. Neurol. Sci. 2000, 180, 52–54. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, J.; Li, S.; Li, Z. Neuroprotective Effects of Genistein in a SOD1-G93A Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. J. Neuroimmune Pharm. 2019, 14, 688–696. [Google Scholar] [CrossRef]
- Chico, L.; Caldarazzo-Ienco, E.; Bisordi, C.; LoGerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial After Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 2018, 17, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Patel, P.; Julien, J.-P. Protective effects of Withania somnifera extract in SOD1G93A mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2018, 309, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, S.; Li, X.; Luo, G.; Li, L.; Le, W. Neuroprotective Effects of (-)-Epigallocatechin-3-gallate in a Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. Neurochem. Res. 2006, 31, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Liu, S.-B.; Zhang, H.-Y.; Zhou, F.-H.; Liu, Y.-X.; Lu, Q.; Yang, L. Antioxidant effects of celastrol against hydrogen peroxide-induced oxidative stress in the cell model of amyotrophic lateral sclerosis. Sheng Li Xue Bao Acta Physiol. Sin. 2017, 69, 751–758. [Google Scholar]
- Yun, Y.C.; Jeong, S.-G.; Kim, S.H.; Cho, G.-W. Reduced sirtuin 1/adenosine monophosphate-activated protein kinase in amyotrophic lateral sclerosis patient-derived mesenchymal stem cells can be restored by resveratrol. J. Tissue Eng. Regen. Med. 2019, 13, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Chu, S.-F.; Li, J.-P.; Zhang, Z.; Yan, J.-Q.; Wen, Z.-L.; Xia, C.-Y.; Mou, Z.; Wang, Z.-Z.; He, W.-B.; et al. Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington’s disease. Acta Pharmacol. Sin. 2015, 36, 311–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Túnez, I.; Montilla, P.; Muñoz, M.D.C.; Feijóo, M.; Salcedo, M. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington’s disease. J. Pineal Res. 2004, 37, 252–256. [Google Scholar] [CrossRef]
- Vidoni, C.; Secomandi, E.; Castiglioni, A.; Melone, M.A.B.; Isidoro, C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem. Int. 2018, 117, 174–187. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Duennwald, M.; Markovic, P.; Wacker, J.L.; Engemann, S.; Roark, M.; Legieiter, J.; Marsh, J.L.; Thompson, L.M.; Lindquist, S.; et al. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum. Mol. Genet. 2006, 15, 2743–2751. [Google Scholar] [CrossRef]
- Fu, J.; Jin, J.; Cichewicz, R.H.; Hageman, S.A.; Ellis, T.K.; Xiang, L.; Peng, Q.; Jiang, M.; Arbez, N.; Hotaling, K.; et al. Trans-(−)-ϵ-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J. Biol. Chem. 2012, 287, 24460–24472. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kumar, A. Possible Neuroprotective Effect of Withania somnifera Root Extract Against 3-Nitropropionic Acid-Induced Behavioral, Biochemical, and Mitochondrial Dysfunction in an Animal Model of Huntington’s Disease. J. Med. Food 2009, 12, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Lee, S.E.; Lee, J.H.; Kim, H.D.; Seo, K.-H.; Kim, D.H.; Han, S.Y. Aster ageratoides Turcz. extract attenuates Alzheimer’s disease-associated cognitive deficits and vascular dementia-associated neuronal death. Anat. Cell Boil. 2020, 53, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Z.; Wu, W.-Y.; Huang, H.; Yin, Y.-Y.; Wu, Y.-Y. Protective effect of bilobalide on learning and memory impairment in rats with vascular dementia. Mol. Med. Rep. 2013, 8, 935–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, W.; Wang, Y. A ginkgo biloba extract promotes proliferation of endogenous neural stem cells in vascular dementia rats. Neural Regen. Res. 2013, 8, 1655–1662. [Google Scholar] [PubMed]
- Demarin, V.; Kes, V.B.; Trkanjec, Z.; Budišić, M.; Pasić, M.B.; Črnac, P.; Budinčević, H. Efficacy and safety of Ginkgo biloba standardized extract in the treatment of vascular cognitive impairment: A randomized, double-blind, placebo-controlled clinical trial. Neuropsychiatr. Dis. Treat. 2017, 13, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.-Q.; Liang, X.-M.; Wu, J.; Zhang, Y.-F.; Zhu, C.-X.; Jiang, X.-J. Treatment with Huperzine A Improves Cognition in Vascular Dementia Patients. Cell Biophys. 2011, 62, 55–58. [Google Scholar] [CrossRef]
- Xing, S.-H.; Zhu, C.-X.; Zhang, R.; An, L. Huperzine A in the Treatment of Alzheimer’s Disease and Vascular Dementia: A Meta-Analysis. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Tang, F.; Guo, S.; Liao, H.; Yu, P.; Wang, L.; Song, X.; Chen, J.; Yang, Q. Resveratrol Enhances Neurite Outgrowth and Synaptogenesis Via Sonic Hedgehog Signaling Following Oxygen-Glucose Deprivation/Reoxygenation Injury. Cell. Physiol. Biochem. 2017, 43, 852–869. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Zhou, L.; Zeng, L.; Yang, Q. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016, 14, 3646–3654. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.-S.; Zheng, P.-D.; Mungur, R.; Zhou, H.-J.; Hassan, M.; Jiang, S.-N. Ginkgolide B promotes the proliferation and differentiation of neural stem cells following cerebral ischemia/reperfusion injury, both in vivo and in vitro. Neural Regen. Res. 2018, 13, 1204–1211. [Google Scholar] [CrossRef]
- Xie, C.J.; Gu, A.P.; Cai, J.; Wu, Y.; Chen, R.C. Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 2018, 8, e00921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Alcântara, G.F.T.; Simões-Neto, E.; Da Cruz, G.M.P.; Nobre, M.E.P.; Neves, K.R.T.; De Andrade, G.M.; Brito, G.; Viana, G.S.D.B. Curcumin reverses neurochemical, histological and immuno-histochemical alterations in the model of global brain ischemia. J. Tradit. Complement. Med. 2016, 7, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, B.; Zeng, Q.; Zhang, Z.; Qian, M.; Chen, J.; Dong, W.-L.; Li, M. Epicatechin Gallate Protects HBMVECs from Ischemia/Reperfusion Injury through Ameliorating Apoptosis and Autophagy and Promoting Neovascularization. Oxidative Med. Cell. Longev. 2019, 2019, 7824684. [Google Scholar] [CrossRef]
- Park, D.J.; Kang, J.B.; Koh, P.O. Epigallocatechin gallate alleviates neuronal cell damage against focal cerebral ischemia in rats. J. Vet. Med. Sci. 2020, 82, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wang, H.; Yang, Y.; Wang, R.; Wang, Y.; Wu, C.; Du, G.-H. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed. Pharm. 2019, 117, 109102. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jiang, Z.; Ning, L.; Zhao, Z.; Yang, N.; Chen, L.; Ma, H.; Li, L.; Fu, Y.; Zhu, H.; et al. Protective HSP70 Induction by Z-Ligustilide against Oxygen–Glucose Deprivation Injury via Activation of the MAPK Pathway but Not of HSF1. Boil. Pharm. Bull. 2015, 38, 1564–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Wang, M.; Zhang, J.; Cai, Q.; Lü, D.; Li, Y.; Dong, Y.; Zhao, T.; Chen, H. The neuroprotection of Sinomenine against ischemic stroke in mice by suppressing NLRP3 inflammasome via AMPK signaling. Int. Immunopharmacol. 2016, 40, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Zhu, Y.; Chen, S.; Zhou, D.; Wang, Y.-Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci. Lett. 2013, 534, 123–127. [Google Scholar] [CrossRef]
- Vaibhav, K.; Shrivastava, P.; Tabassum, R.; Khan, A.; Javed, H.; Ahmed, E.; Islam, F.; Safhi, M.M.; Islam, F. Delayed administration of zingerone mitigates the behavioral and histological alteration via repression of oxidative stress and intrinsic programmed cell death in focal transient ischemic rats. Pharmacol. Biochem. Behav. 2013, 113, 53–62. [Google Scholar] [CrossRef]
- Tabassum, R.; Vaibhav, K.; Shrivastava, P.; Khan, A.; Ahmed, M.E.; Ashafaq, M.; Khan, M.B.; Islam, F.; Safhi, M.M.; Islam, F. Perillyl alcohol improves functional and histological outcomes against ischemia–reperfusion injury by attenuation of oxidative stress and repression of COX-2, NOS-2 and NF-κB in middle cerebral artery occlusion rats. Eur. J. Pharmacol. 2015, 747, 190–199. [Google Scholar] [CrossRef]
- Vaibhav, K.; Shrivastava, P.; Javed, H.; Khan, A.; Ahmed, E.; Tabassum, R.; Khan, M.M.; Khuwaja, G.; Islam, F.; Siddiqui, M.S.; et al. Piperine suppresses cerebral ischemia–reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-κB in middle cerebral artery occlusion rat model. Mol. Cell. Biochem. 2012, 367, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Park, S.-Y. Baicalein prevents human prion protein-induced neuronal cell death by regulating JNK activation. Int. J. Mol. Med. 2014, 35, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.-K.; Moon, M.-H.; Bae, B.-C.; Lee, Y.-J.; Seol, J.-W.; Kang, H.-S.; Kim, J.-S.; Kang, S.-J.; Park, S.-Y. Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci. Res. 2012, 73, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Lee, J.-H.; Lee, Y.-J.; Park, S.-Y. Hinokitiol protects primary neuron cells against prion peptide-induced toxicity via autophagy flux regulated by hypoxia inducing factor-1. Oncotarget 2016, 7, 29944–29957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, J.-Y.; Kim, S.; Song, K.; Kwon, J. Rutin Alleviates Prion Peptide-Induced Cell Death Through Inhibiting Apoptotic Pathway Activation in Dopaminergic Neuronal Cells. Cell. Mol. Neurobiol. 2014, 34, 1071–1079. [Google Scholar] [CrossRef]
- Minami, S.S.; Min, S.-W.; Krabbe, G.; Wang, C.; Zhou, Y.; Asgarov, R.; Li, Y.; Martens, L.H.; Elia, L.P.; Ward, M.E.; et al. Progranulin protects against amyloid β deposition and toxicity in Alzheimer’s disease mouse models. Nat. Med. 2014, 20, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Matsumura, S.; Yoshioka, Y.; Ueno, Y.; Matsuda, H. Screening of β-secretase and acetylcholinesterase inhibitors from plant resources. J. Nat. Med. 2014, 69, 123–129. [Google Scholar] [CrossRef]
- Maguire, E.; Haslett, L.J.; Welton, J.L.; Lloyd-Evans, E.; Goike, J.; Clark, E.H.; Knifton, H.R.; Shrestha, R.; Wager, K.; Webb, R.; et al. Effects of curcumin nanoformulations on cellular function in Niemann-Pick disease type C astrocytes. bioRxiv 2017, 135830. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Liu, K.; Swaroop, M.; Porter, F.D.; Sidhu, R.; Finkes, S.; Ory, D.S.; Marugan, J.J.; Xiao, J.; Southall, N.T.; et al. δ-Tocopherol Reduces Lipid Accumulation in Niemann-Pick Type C1 and Wolman Cholesterol Storage Disorders. J. Boil. Chem. 2012, 287, 39349–39360. [Google Scholar] [CrossRef] [Green Version]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and Quercetin Affect the Cholesterol Absorption Mediated by Epithelial Cholesterol Transporter Niemann–Pick C1-Like 1 in Caco-2 Cells and Rats. PLoS ONE 2014, 9, e97901. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-M.; Lin, C.-H.; Wu, Y.R.; Yen, C.-Y.; Huang, Y.-T.; Lin, J.-L.; Lin, C.-Y.; Chen, W.-L.; Chao, C.-Y.; Lee-Chen, G.-J.; et al. Lactulose and Melibiose Inhibit α-Synuclein Aggregation and Up-Regulate Autophagy to Reduce Neuronal Vulnerability. Cells 2020, 9, 1230. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-C.; Lin, C.-H.; Tao, Y.-C.; Yang, J.-M.; Hsu, K.-C.; Huang, Y.-J.; Huang, S.-H.; Kung, P.-J.; Chen, W.-L.; Wang, C.-M.; et al. The potential of lactulose and melibiose, two novel trehalase-indigestible and autophagy-inducing disaccharides, for polyQ-mediated neurodegenerative disease treatment. NeuroToxicology 2015, 48, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Jeong, H.; Ham, Y.; Jo, Y.H.; Choi, M.; Kang, M.; Son, B.; Choi, S.; Ryu, H.W.; Kim, J.; et al. Improvement of spinal muscular atrophy via correction of the SMN2 splicing defect by Brucea javanica (L.) Merr. extract and Bruceine, D. Phytomedicine 2019, 65, 153089. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-Y.; Jong, Y.-J.; Tsai, H.-H.; Tseng, Y.-T.; An, L.-M.; Lo, Y.-C. Triptolide increases transcript and protein levels of survival motor neurons in human SMA fibroblasts and improves survival in SMA-like mice. Br. J. Pharmacol. 2012, 166, 1114–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leverenz, J.B.; Quinn, J.F.; Zabetian, C.; Zhang, J.; Montine, K.S.; Montine, T.J. Cognitive impairment and dementia in patients with Parkinson disease. Curr. Top. Med. Chem. 2009, 9, 903–912. [Google Scholar]
- Zecca, L.; Shima, T.; Stroppolo, A.; Goj, C.; Battiston, G.; Gerbasi, R.; Sarna, T.; Swartz, H. Interaction of neuromelanin and iron in substantia nigra and other areas of human brain. Neuroscience 1996, 73, 407–415. [Google Scholar] [CrossRef]
- Hoang, Q.Q. Pathway for Parkinson disease. Proc. Natl. Acad. Sci. USA 2014, 111, 2402–2403. [Google Scholar] [CrossRef] [Green Version]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Albin, R.L. Parkinson’s Disease: Background, Diagnosis, and Initial Management. Clin. Geriatr. Med. 2006, 22, 735–751. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.X.; Wood, N.W.; Latchman, D.S. Molecular basis of Parkinson’s disease. NeuroReport 2009, 20, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, H. Modern treatment in Parkinson’s disease, a personal approach. J. Neural Transm. 2015, 123, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Peschanski, M.; Defer, G.; Nguyen, J.P.; Ricolfi, F.; Monfort, J.C.; Hantraye, P.; Jeny, R.; Degos, J.D.; Cesaro, P.; Rémy, P.; et al. Bilateral motor improvement and alteration of L-dopa effect in two patients with Parkinson’s disease following intrastriatal transplantation of foetal ventral mesencephalon. Brain 1994, 117, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Nabavi, S.F.; Daglia, M.; Martinoli, M.-G. Epigallocatechin-3-Gallate, a Promising Molecule for Parkinson’s Disease? Rejuvenation Res. 2015, 18, 257–269. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Siew, C.J.; Mitra, N.K.; Kumari, M. Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci. Lett. 2011, 500, 139–143. [Google Scholar] [CrossRef]
- El-Horany, H.E.; El-Latif, R.N.A.; Elbatsh, M.M.; Emam, M.N. Ameliorative Effect of Quercetin on Neurochemical and Behavioral Deficits in Rotenone Rat Model of Parkinson’s Disease: Modulating Autophagy (Quercetin on Experimental Parkinson’s Disease). J. Biochem. Mol. Toxicol. 2016, 30, 360–369. [Google Scholar] [CrossRef]
- Zhu, J.T.T.; Choi, R.C.Y.; Chu, G.K.Y.; Cheung, A.W.H.; Gao, Q.T.; Li, J.; Jiang, Z.Y.; Dong, T.T.X.; Tsim, K.W.K. Flavonoids Possess Neuroprotective Effects on Cultured Pheochromocytoma PC12 Cells: A Comparison of Different Flavonoids in Activating Estrogenic Effect and in Preventing β-Amyloid-Induced Cell Death. J. Agric. Food Chem. 2007, 55, 2438–2445. [Google Scholar] [CrossRef]
- Mirahmadi, S.-M.-S.; Shahmohammadi, A.; Rousta, A.-M.; Azadi, M.-R.; Fahanik-Babaei, J.; Baluchnejadmojarad, T.; Roghani, M. Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine 2018, 104, 151–159. [Google Scholar] [CrossRef]
- Lou, H.; Jing, X.; Wei, X.; Shi, H.; Ren, D.; Zhang, X. Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 2014, 79, 380–388. [Google Scholar] [CrossRef]
- Kim, H.-J.; Song, J.Y.; Park, H.J.; Park, H.-K.; Yun, D.H.; Chung, J.-H. Naringin Protects against Rotenone-induced Apoptosis in Human Neuroblastoma SH-SY5Y Cells. Korean J. Physiol. Pharmacol. 2009, 13, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.-C.; Ko, C.H.; Tseng, S.-W.; Tsai, S.-H.; Chen, Y.-C. Structurally related antitumor effects of flavanones in vitro and in vivo: Involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol. 2004, 197, 84–95. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Jung, K.K.; Cho, J.Y.; Rhee, M.H.; Hong, S.; Kwon, M.; Kim, S.H.; Kang, S.Y. Neuroprotective effect of curcumin is mainly mediated by blockade of microglial cell activation. Die Pharm. 2007, 62, 937–942. [Google Scholar]
- Wang, M.S.; Boddapati, S.; Emadi, S.; Sierks, M.R. Curcumin reduces α-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neurosci. 2010, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragicevic, N.; Smith, A.; Lin, X.; Yuan, F.; Copes, N.; Delic, V.; Tan, J.; Cao, C.; Shytle, R.D.; Bradshaw, P.C. Green Tea Epigallocatechin-3-Gallate (EGCG) and Other Flavonoids Reduce Alzheimer’s Amyloid-Induced Mitochondrial Dysfunction. J. Alzheimer’s Dis. 2011, 26, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Lau, W.K.-W.; Huie, M.J.; Ho, Y.-S.; Yu, M.-S.; Lai, C.S.-W.; Wang, M.; Yuen, W.-H.; Lam, W.H.; Chan, T.H.; et al. A pro-drug of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) prevents differentiated SH-SY5Y cells from toxicity induced by 6-hydroxydopamine. Neurosci. Lett. 2010, 469, 360–364. [Google Scholar] [CrossRef] [Green Version]
- Leaver, K.R.; Allbutt, H.N.; Creber, N.J.; Kassiou, M.; Henderson, J.M. Oral pre-treatment with epigallocatechin gallate in 6-OHDA lesioned rats produces subtle symptomatic relief but not neuroprotection. Brain Res. Bull. 2009, 80, 397–402. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Kostrzewa, R.M. Neurotoxin Mechanisms and Processes Relevant to Parkinson’s Disease: An Update. Neurotox. Res. 2015, 27, 328–354. [Google Scholar] [CrossRef]
- Hedya, S.A.; Safar, M.M.; Bahgat, A.K. Cilostazol Mediated Nurr1 and Autophagy Enhancement: Neuroprotective Activity in Rat Rotenone PD Model. Mol. Neurobiol. 2018, 55, 7579–7587. [Google Scholar] [CrossRef]
- Ferretta, A.; Gaballo, A.; Tanzarella, P.; Piccoli, C.; Capitanio, N.; Nico, B.; Annese, T.; Di Paola, M.; Dell’Aquila, C.; De Mari, M.; et al. Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 902–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quik, M.; Perez, X.A.; Bordia, T. Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov. Disord. 2012, 27, 947–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendouei, F.; Moghaddam, H.S.; Mohammadi, M.R.; Taslimi, N.; Rezaei, F.; Akhondzadeh, S. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: A double-blind and placebo-controlled randomized trial. J. Clin. Pharm. Ther. 2019, 45, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Kolankaya, D. Inhibiting inducible nitric oxide synthase with rutin reduces renal ischemia/reperfusion injury. Can. J. Surg. 2013, 56, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—A mini-review. Life Sci. 2014, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, M.; Wang, X.; Wang, Y.; Duan, C.; Gao, G.; Lu, L.; Wu, X.; Wang, X.; Yang, H. Piperine induces autophagy by enhancing protein phosphotase 2A activity in a rotenone-induced Parkinson’s disease model. Oncotarget 2016, 7, 60823–60843. [Google Scholar] [CrossRef] [Green Version]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Erratum: Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 1–19. [Google Scholar]
- Boillée, S.; Yamanaka, K.; Lobsiger, C.S.; Copeland, N.G.; Jenkins, N.A.; Kassiotis, G.; Kollias, G.; Cleveland, D.W. Onset and Progression in Inherited ALS Determined by Motor Neurons and Microglia. Science 2006, 312, 1389–1392. [Google Scholar] [CrossRef] [Green Version]
- Al-Chalabi, A.; Berg, L.H.V.D.; Veldink, J. Gene discovery in amyotrophic lateral sclerosis: Implications for clinical management. Nat. Rev. Neurol. 2016, 13, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Simpson, E.P.; Yen, A.A.; Appel, S.H. Oxidative Stress: A common denominator in the pathogenesis of amyotrophic lateral sclerosis. Curr. Opin. Rheumatol. 2003, 15, 730–736. [Google Scholar] [CrossRef]
- Grosskreutz, J.; Bosch, L.V.D.; Keller, B.U. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium 2010, 47, 165–174. [Google Scholar] [CrossRef]
- McGeer, P.L.; McGeer, E.G. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 2002, 26, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Swash, M.; Schwartz, M.S. What do we really know about amyotrophic lateral sclerosis? J. Neurol. Sci. 1992, 113, 4–16. [Google Scholar] [CrossRef]
- Niebroj-Dobosz, I.; Janik, P. Amino acids acting as transmitters in amyotrophic lateral sclerosis (ALS). Acta Neurol. Scand. 1999, 100, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Semmler, S.; Gagné, M.; Garg, P.; Pickles, S.R.; Baudouin, C.; Hamon-Keromen, E.; Destroismaisons, L.; Khalfallah, Y.; Chaineau, M.; Caron, E.; et al. TNF receptor-associated factor 6 interacts with ALS-linked misfolded superoxide dismutase 1 and promotes aggregation. J. Boil. Chem. 2020, 295, 3808–3825. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. Pharmacologic Approaches to the Treatment of Amyotrophic Lateral Sclerosis. BioDrugs 2005, 19, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Patel, P.; Rahimian, R.; Phaneuf, D.; Julien, J.P. Withania somnifera Reverses Transactive Response DNA Binding Protein 43 Proteinopathy in a Mouse Model of Amyotrophic Lateral Sclerosis/Frontotemporal Lobar Degeneration. Neurother. J. Am. Soc. Exp. NeuroTher. 2017, 14, 447–462. [Google Scholar] [CrossRef] [Green Version]
- Ivanenkov, Y.A.; Balakin, K.V.; Lavrovsky, Y. Small Molecule Inhibitors of NF-B and JAK/STAT Signal Transduction Pathways as Promising Anti-Inflammatory Therapeutics. Mini Rev. Med. Chem. 2011, 11, 55–78. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, W.; Wang, H.; Yang, Y.; Wang, T.; Wang, S.; Wang, X.; Wang, Y.; Feng, H. γ-Oryzanol mitigates oxidative stress and prevents mutant SOD1-Related neurotoxicity in Drosophila and cell models of amyotrophic lateral sclerosis. Neuropharmacology 2019, 160, 107777. [Google Scholar] [CrossRef]
- Szlachcic, W.J.; Switonski, P.M.; Krzyzosiak, W.J.; Figlerowicz, M.; Figiel, M. Huntington disease iPSCs show early molecular changes in intracellular signaling, the expression of oxidative stress proteins and the p53 pathway. Dis. Model. Mech. 2015, 8, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxidative Med. Cell. Longev. 2016, 2016, 1–15. [Google Scholar] [CrossRef]
- Friedlander, R.M. Apoptosis and Caspases in Neurodegenerative Diseases. N. Engl. J. Med. 2003, 348, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Suwanjang, W.; Govitrapong, P.; Chetsawang, B.; Phansuwan-Pujito, P. The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cultured cells. J. Pineal Res. 2010, 48, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.; Lorenzen, N.; Otzen, D. Interactions between misfolded protein oligomers and membranes: A central topic in neurodegenerative diseases? Biochim. Biophys. Acta Biomembr. 2015, 1848, 1897–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, R.P.; Giorgini, F. Modeling Huntington disease in yeast: Perspectives and future directions. Prion 2011, 5, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moumné, L.; Betuing, S.; Caboche, J. Multiple Aspects of Gene Dysregulation in Huntington’s Disease. Front. Neurol. 2013, 4, 127. [Google Scholar] [CrossRef] [Green Version]
- Szegő, É.M.; Outeiro, T.F.; Kazantsev, A.G. Sirtuins in Brain and Neurodegenerative Disease. Introd. Rev. Sirtuins Biol. Aging Dis. 2018, 175–195. [Google Scholar] [CrossRef]
- Túnez, I.; Tasset, I.; La Cruz, V.P.-D.; Santamaría, A. 3-Nitropropionic Acid as a Tool to Study the Mechanisms Involved in Huntington’s Disease: Past, Present and Future. Molecules 2010, 15, 878–916. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.; Erkinjuntti, T.; Reisberg, B.; Román, G.; Sawada, T.; Pantoni, L.; Bowler, J.V.; Ballard, C.; DeCarli, C.; Gorelick, P.B.; et al. Vascular cognitive impairment. Lancet Neurol. 2003, 2, 89–98. [Google Scholar] [CrossRef]
- Hicks, A.; Jolkkonen, J. Challenges and possibilities of intravascular cell therapy in stroke. Acta Neurobiol. Exp. 2009, 69, 1–11. [Google Scholar]
- Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982, 239, 57–69. [Google Scholar] [CrossRef]
- Abas, F.; Alkan, T.; Goren, B.; Taskapilioglu, O.; Sarandol, E.; Tolunay, S. Neuroprotective effects of postconditioning on lipid peroxidation and apoptosis after focal cerebral ischemia/reperfusion injury in rats. Turk. Neurosurg. 2010, 20, 1–8. [Google Scholar] [PubMed]
- Jin, W.-N.; Shi, S.X.-Y.; Li, Z.; Li, M.; Wood, K.; Gonzales, R.J.; Liu, Q. Depletion of microglia exacerbates postischemic inflammation and brain injury. Br. J. Pharmacol. 2017, 37, 2224–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, G.; Lyu, M.; Wang, Y.; He, S.; Liu, X.; Ni, J.; Li, L.; Fan, G.; Han, J.; Gao, X.; et al. Ginkgo Flavonol Glycosides or Ginkgolides Tend to Differentially Protect Myocardial or Cerebral Ischemia–Reperfusion Injury via Regulation of TWEAK-Fn14 Signaling in Heart and Brain. Front. Pharmacol. 2019, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, W.; Sun, Y.J.; Hu, M.; Li, F.; Zhu, D. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood–brain barrier damage. Eur. J. Pharmacol. 2007, 561, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Zhonghang, X.; Keyan, C.; Tongtong, L.; Wanshu, G.; Xu, Z.-X. Epigallocatechin-3-Gallate Reduces Neuronal Apoptosis in Rats after Middle Cerebral Artery Occlusion Injury via PI3K/AKT/eNOS Signaling Pathway. BioMed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Aryal, P.; Kim, K.; Park, P.-H.; Ham, S.; Cho, J.; Song, K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014, 281, 4644–4658. [Google Scholar] [CrossRef]
- Wu, P.-F.; Zhang, Z.; Wang, F.; Chen, J. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol. Sin. 2010, 31, 1523–1531. [Google Scholar] [CrossRef]
- Xu, H.; Tang, W.; Du, G.-H.; Kokudo, N. Targeting apoptosis pathways in cancer with magnolol and honokiol, bioactive constituents of the bark of Magnolia officinalis. Drug Discov. Ther. 2011, 5, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Barron, R. Infectious prions and proteinopathies. Prion 2017, 11, 40–47. [Google Scholar] [CrossRef]
- Marín-Moreno, A.; Fernández-Borges, N.; Espinosa, J.C.; Andréoletti, O.; Torres, J.M. Transmission and Replication of Prions. Prog. Mol. Biol. Transl. Sci. 2017, 150, 181–201. [Google Scholar] [CrossRef]
- Ma, J.; Ma, J. Prion disease and the ‘protein-only hypothesis’. Essays Biochem. 2014, 56, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, J. Immunotherapy against Prion Disease. Pathogens 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baiardi, S.; Rossi, M.; Capellari, S.; Parchi, P. Recent advances in the histo-molecular pathology of human prion disease. Brain Pathol. 2019, 29, 278–300. [Google Scholar] [CrossRef] [PubMed]
- Bott, N.; Radke, A.; Stephens, M.L.; Kramer, J.H. Frontotemporal dementia: Diagnosis, deficits and management. Neurodegener. Dis. Manag. 2014, 4, 439–454. [Google Scholar] [CrossRef] [Green Version]
- Kelley, R.E.; El-Khoury, R. Frontotemporal Dementia. Neurol. Clin. 2016, 34, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Mohandas, E.; Rajmohan, V. Frontotemporal dementia: An updated overview. Indian J. Psychiatry 2009, 51, S65–S69. [Google Scholar]
- Miller, B.L. Frontotemporal Dementia. Front. Dement. 2014, 35, 339–374. [Google Scholar] [CrossRef] [Green Version]
- Kertesz, A.; Muñoz, D. Pick’s Disease, Frontotemporal Dementia, and Pick Complex. Arch. Neurol. 1998, 55, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Frederick, J. Pick disease: A brief overview. Arch. Pathol. Lab. Med. 2006, 130, 1063–1066. [Google Scholar]
- Minami, S.S.; Shen, V.; Le, D.; Krabbe, G.; Asgarov, R.; Liberty, P.-C.; Lee, C.-H.; Li, J.; Donnelly-Roberts, D.; Gan, L. Reducing inflammation and rescuing FTD-related behavioral deficits in progranulin-deficient mice with α7 nicotinic acetylcholine receptor agonists. Biochem. Pharmacol. 2015, 97, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.; Banerjee, R.; Thomas, B.; Zhou, P.; Qian, L.; Jia, T.; Ma, X.; Ma, Y.; Iadecola, C.; Beal, M.F.; et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J. Exp. Med. 2009, 207, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caires, S.; Steenkamp, V. Use of Yokukansan (TJ-54) in the treatment of neurological disorders: A review. Phytother. Res. 2010, 24, 1265–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Hayashida, H.; Furukawa, H.; Takamatsu, J. Pilot study of pharmacological treatment for frontotemporal dementia: Effect of Yokukansan on behavioral symptoms. Psychiatry Clin. Neurosci. 2010, 64, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar Ataxia. Nat. Rev. Dis. Primers 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Schöls, L.; Bauer, P.; Schmidt, T.; Schulte, T.; Riess, O. Autosomal dominant cerebellar ataxias: Clinical features, genetics, and pathogenesis. Lancet Neurol. 2004, 3, 291–304. [Google Scholar] [CrossRef]
- Groth, C.L.; Berman, B.D. Spinocerebellar Ataxia 27: A Review and Characterization of an Evolving Phenotype. Tremor Other Hyperkinetic Mov. 2018, 8, 534. [Google Scholar] [CrossRef]
- Sullivan, R.; Yau, W.Y.; O’Connor, E.; Houlden, H. Spinocerebellar ataxia: An update. J. Neurol. 2018, 266, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Arch. Neurol. 2011, 68, 979. [Google Scholar] [CrossRef] [Green Version]
- Farrar, M.A.; Kiernan, M.C. The Genetics of Spinal Muscular Atrophy: Progress and Challenges. Neurotherapeutics 2014, 12, 290–302. [Google Scholar] [CrossRef]
- Rao, V.K.; Kapp, D.; Schroth, M. Gene Therapy for Spinal Muscular Atrophy: An Emerging Treatment Option for a Devastating Disease. J. Manag. Care Spéc. Pharm. 2018, 24, S3–S16. [Google Scholar] [CrossRef]
- Singh, R.N.; Singh, N.N. Mechanism of Splicing Regulation of Spinal Muscular Atrophy Genes. Adv. Neurobiol. 2018, 20, 31–61. [Google Scholar] [CrossRef]
- Fuller, H.R.; Gillingwater, T.H.; Wishart, T.M. Commonality amid diversity: Multi-study proteomic identification of conserved disease mechanisms in spinal muscular atrophy. Neuromuscul Disord. 2016, 26, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Disease | Plant | Natural Compound | In-Vitro/In-Vivo Models/Human Trial | Dose & Route | Mode of Action | References | |
|---|---|---|---|---|---|---|---|
| Alzheimer’s Disease | Curcuma longa | Curcumin | Acrolein exposed HT22 murine hippocampal neuronal cells | 5 μg/mL/30 min | ↓ AD-like pathologies, MDA level, ↑ levels of GSH, SOD, ↓ metalloprotease, APP, β-secretase, ↑BDNF/TrkB signaling | [27] | |
| p25 Transgenic Mouse model | 4 g/kg for 12 weeks; po | ↓ tau, amyloid accumulation, ↑ proinflammatory cytokines | [29] | ||||
| Pilot study with curcumin and ginkgo on 34 possible or probable AD patients | 1 g/day, 4 g/day 120 mg/day ginkgo leaf extract for 6 months | No significant difference in Mini-Mental State Examination scores or plasma Aβ40 levels between 0 and 6 months. No side effect of curcumin | [30] | ||||
| Longvida | 40 subjects: AD, MCI, healthy | 20 g/day for 7 days | Retinal imaging of Aβ plagues differentiated between AD and non-AD subjects | [31] | |||
| Many plants | Apigenin | Copper induced SHSY5Y cells | 10.0 mM for 24 h | ↑antioxidation, mitochondrion protection, ↑MAPK signaling | [32] | ||
| APP/PS1 double transgenic mouse model | 40 mg/kg for 5 day/week | ↑behavior, ↓Aβ burden, ↑ERK/CREB/BDNF pathway, ↓oxidative stress | [33] | ||||
| Glycine max | Genistein | Rat hippocampal neuronal cells | 0.4 μg/mL | ↑ α-secretase, ↓ β-secretase, ↑PKC signaling pathway | [41] | ||
| Bilateral hippocampal Aβ25–35 injected rat Model | 90 mg/kg for 42 days | ↓ p-tau, CALM, CAMKK1, p-CAMK4 protein, escape latency | [42] | ||||
| Ginkgo biloba | Ginkgolides A or B | Mouse primary cortical neurons | 100 or 300 µM/1.5 h | ↓ AMPA- and NMDA-induced depolarization, ↓JNK phosphorylation | [43] | ||
| TgAPP/PS1 mice | 100 mg/kg for 1 month | ↑ cell proliferation in the hippocampus, ↓ Aβ oligomers and ↑ pCREB levels | [44] | ||||
| Many plants | Luteolin | ICV-STZ rat model | 10 and 20 mg/kg | ↑ escape latency, ↑ thickness of CA1 pyramidal layer | [48] | ||
| Many plants | Melatonin | murine N2a neuroblastoma cells | 10 µM | ↑ cell viability, ↓ cell blebbing and retraction, ↓ LPO, intracellular Ca+2 | [49] | ||
| 3xTg-AD transgenic mice | 10 mg/kg/day in drinking water for 1 month | ↑ anxiety and depression like behaviors, ↑ GST and complexin-1 | [50] | ||||
| 157 AD patients | 10 mg melatonin | ↑ nocturnal total sleep time, ↓ wake after sleep | [51] | ||||
| Citrus junos | Naringenin | Aβ25-35 treated PC-12 cells | 0.4 µM for 2 h | ↑ cell viability, ↑ ER-mediated PI3K/Akt signaling pathway, ↓ caspase-3 | [52] | ||
| AlCl3+D-gal rat model | 50 mg/kg for 2 weeks po | ↑ behavioral parameters, ↑ antioxidant enzymes, ↓ LPO, AChE, ↑ ACh levels, 5-HT levels and DA levels, ↓ DOPAC levels | [53] | ||||
| Piper nigrum | Piperine | ICV-STZ C57BL/6 mice | 10 mg/kg/22 days | ↑ behavioral parameters, ↑ antioxidant enzymes, ↓ LPO, ↑ ARG1, CD206, NE, DA, 5-HT and GABA levels and ↓ glutamate level in hippocampus, ↓ CD86 and iNOS, | [54] | ||
| Many plants | Quercetin | fAβ 1-40 insulted hBMECs | 0.3, 3 and 30 µmol/L | ↑ cell viability, ↑ γ-GT and ALP, ↓oxidative stress, ↑ barrier function | [55] | ||
| triple transgenic AD (3xTg-AD) mice | 25 mg/kg every 2 days for 3 months | ↑ neuronal population, ↓ β-amyloid accumulation, tau accumulation, astrogliosis and microgliosis, ↑behavior parameters | [56] | ||||
| Grapes and other plants | Resveratrol | Aβ1-42-treated PC12 cells | 3 μM | ↑ cell viability, ↑ mitophagy, ↓ apoptosis and ROS | [57] | ||
| AβPPswe/PS1dE9mouse model | 16 mg/kg/day | ↑ behavior, ↓ plague pathology, ↑ p-AMPK/LKB pathway, ↓ SIRT-1, ↑ IL1β and TNF | [58] | ||||
| 199 patients’ mild to moderate dementia due to AD. | 500 mg once daily with 500 mg increments every 13 weeks leading to 1000 mg twice daily. | ↑ CSF and plasma Aβ 40 in placebo, preserves brain barrier integrity | [59] | ||||
| Nigella sativa | Thymoquinone | Aβ25-35-treated PC12 cells | 4 μM for 24 h | ↑ cell viability, ↓ LDH levels, ↓ TBARS, ↑ GSH, antioxidant enzymes, ↓ ROS, AChE, NO levels, ↑ MMP, ↓ iNOS expression | [60] | ||
| Allium sativum | s-allyl cysteine (SAC) | ICV-STZ mice model | 30 mg/kg i.p. for 15 days | ↑behavior, ↑ GSH, antioxidant enzymes, ↓ LPO, ↑ Bcl2 and ↓ p53 levels | [61] | ||
| D-galactose (DG) mice | 1 g/L in drinking water for 7 weeks | ↓ Aβ1-40 and Aβ1-42, ↓ APP and BACE1 expression, ↑ PKC activity | [62] | ||||
| Many plants | Eucalyptol | Aβ25-35-treated PC12 cells | 10 μM/24 h | ↓LDH levels, ↓ TBARS, ↑ GSH, antioxidant enzymes, ↓ ROS, AChE, NO levels, ↓ cytokines, ↓ NF-κB, COX-2, iNOS, ↑cell viability | [63] | ||
| Silybum marianum | silibinin | APPswe/PS1dE9 double-transgenic mice | 100 mg/kg/15 days | ↑ behavior in MWM, ↓ amyloid plaque burden, ↓ gut bacterial | [64] | ||
| Aβ1-42 model of rats | 25, 50 and 100 mg/kg for 10 days | ↓ depression, anxiety behavior, ↓ neuronal damage, ↑ BDNF and TrkB expression, ↑ autophagy | [65] | ||||
| Parkinson’s Disease | Many plants | Quercetin | The mouse dopaminergic MN9D cell line | 10 and 30 µM quercetin for 24 h | ↑ PKD1 pro-survival signaling, Akt and CREB phosphorylation, BDNF expression, mitochondrial biogenesis | [66] | |
| 6-OHDA model of rats | 100, 200, 300 mg/kg for 14 days | ↑ motor and non-motor deficits, antioxidant enzyme activities, ↑ neuron density in hippocampus, ↓ AChE | [67] | ||||
| Glycine max | Genistein | SH-SY5Y cells overexpressing A53T mutant α-synuclein | 20 μM for 24 h | ↑ cell viability, ↓ MDA content, ↑ GSH, ATP and Na+K+ATPase levels, ↓ apoptosis | [68] | ||
| Transgenic Drosophila Model expressing human α-synuclein | 10, 20, 30, and 40 µM for 24 days | ↑ life expectancy, ↓ loss of climbing ability, ↓ oxidative stress, ↑ dopamine content | [69] | ||||
| Grapefruit and other citrus fruits | Naringin | MPP+-induced Parkinson’s disease rat model | 80 mg/kg for 4 days | ↑ GDNF expression and mTORC1, ↓ TNF-α | [70] | ||
| Curcuma longa | Curcumin | MPP+-induced SH-SY5Y cell | 40 µM for 24 h | ↑ cell viability, DA, Bcl-xl level, ↓ caspase 3, Bax level and HSP-90 levels | [71] | ||
| Lipopolysaccharide-induced PD rat model | 40 mg/kg i.p. for 21 days | ↓ GFAP, NFκB, TNF-α, IL-1β, IL-1α, iNOS, oxidative stress, α-synuclein aggregates, apoptotic markers | [72] | ||||
| Green tea | Epigallocatechin gallate (EGCG) | Rotenone treated SH-SY5Y cells | 25 or 50 μM for 24 h | ↓ caspase-3 and apoptosis, ↑ cell viability, SOD | [73] | ||
| MPTP mouse model | 25 and 50 mg/kg for 20 days | ↑ behavior, TH-positive neurons, ↓ TNF-α and IL-6 | [74] | ||||
| 480 PD patients, randomized, double blind | three dosage groups of green tea polyphenol and one placebo control group | Delay in motor function progression, ↑ cognition, mood and quality of life | ClinicalTrials.gov identifier: NCT00461942 | ||||
| Grapes and other plants | Resveratrol | 6-OHDA treated PC 12 cells | 50 μM for 24 h | ↑cell viability, MMP, ↓ apoptosis, CXCR4 protein levels | [75] | ||
| MPTP mouse model | 50 mg/kg for 3 weeks | ↑ TH+ cells and miR-129, ↓ MALAT1, SNCA and apoptosis | [76] | ||||
| 20 double blind, crossover, randomized, Placebo controlled phase 1 study | BIA 6-512 (trans-resveratrol) 25 mg dose, 50 mg dose, 100 mg dose | To study BIA 6-512 effect on levodopa pharmacokinetics when administered with levodopa/benserazide | ClinicalTrials.gov Identifier: NCT03091543 | ||||
| Many plants | Rutin | 6-OHDA in PC-12 cells | 10, 50, and 100 µM for 8 h | ↑cell viability, catalase, SOD, GPx, GSH, ↓ MDA | [77] | ||
| 6-OHDA-induced PD rat model | 25 mg/kg for 21 days | ↑ behavior activities, GSH and dependent enzymes, DA and its metabolites, protein carbonyl, ↓ TBARS, H2O2, NO level, TNF-α and IL-1β | [78] | ||||
| Citrus trees | Hesperidin | 6-OHDA-induced PD mice model | 50 mg/kg for 28 days | ↑ behavior activities, GSH and dependent enzymes, SOD, Catalase, DA and its metabolites ↓ROS | [79] | ||
| Piper nigrum | Piperine | 6-OHDA-induced PD mice model | 10 mg/kg for 15 days | ↑ behavior activities, GSH and dependent enzymes, SOD, Catalase, ↓ LPO, Caspase-3 and Caspase-9, TNF-α, and IL-1β | [80] | ||
| Amyotrophic lateral sclerosis | Panax and Eleutherococcus | Ginseng root | SOD1-G93A Transgenic Mouse | 40, 80 mg/kg | ↑ onset to clinical signs and surrogate death | [81] | |
| Genistein | SOD1-G93A Transgenic Mouse | 16 mg/kg 2 times per day | ↑ longevity, ↓ ALS symptoms, ↑ motor neurons, ↓ TLR2, TLR4, and NF-kB, p65 levels, IL-1β, IL-6, TNF-α levels | [82] | |||
| Curcuma longa | Brainoil, curcumin supplement | 42 randomised ALS patients | 600 mg/day for 3 months | ↓ disease progression, AOPPs, oxidative damage, ↑ aerobic metabolism | [83] | ||
| Withania somnifera | Withania somnifera extract | SOD1-G93A Transgenic Mouse | 5 mg of root powder p.o. | ↑ longevity, motor performance, Hsp-70, Hsp-60 and Hsp-27, motor neurons, ↓ misfolded SOD1 protein, inflammation | [84] | ||
| Green tea | EGCG | SOD1-G93A Transgenic Mouse | 10 mg/kg body | ↓ disease onset, ↑ survival, motor neurons, ↓ activated microglia, NF-kB and caspase-9 | [85] | ||
| Tripterygium wilfordii and Celastrus regelii | Celastrol | SOD1G93A transfected NSC34 cells | 50 nmol/L for 4 h | ↑ cell viability, ↓ MDA, ↑ mRNA expressions of GCLC and GST, ERK1/2 and Akt | [86] | ||
| Grapes and other plants | Resveratrol | BM-MSCs derived from ALS patients | 1 μM for 12 h | ↑ Neuro-progenitor markers, nestin, NF-M, Tuj-1, and Map-2, AMPK/SIRT1 signaling | [87] | ||
| Huntington’s Disease | Panax ginseng | Protopanaxatriol | 3-NP-induced HD | 5, 10, and 20 mg/kg, po | ↑ weight, locomotor activity, antioxidant enzymes, HO-1, NQO1, ↓ ROS | [88] | |
| Many plants | melatonin | 3-NP-induced HD | 1 mg/kg for 8 days | ↓ LPO, protein carbonyl, ↑ SOD and succinate dehyrogenase | [89] | ||
| 20 HD gene carrier subjects | 5 mg/day, 30 min before bedtime/month | To improve sleep quality in HD gene carriers | ClinicalTrials.gov Identifier: NCT04421339 | ||||
| Grapes and other plants | Resveratrol | SH-SY5Y cells hyper-expressing the mutant polyQ Huntingtin (polyQ-Htt) protein | 100 µM for 24 h | ↑ cell viability, autophagy, autophagy degradation of mutant Huntingtin, ↓ ROS | [90] | ||
| Double blind, randomized controlled 102 early affected HD patients | 800 mg/day for 1 year | To evaluate resveratrol effect on caudate volume in HD patients | ClinicalTrials.gov Identifier: NCT02336633 | ||||
| Green tea | EGCG | Cell culture, yeast model and HD transgenic flies | Different doses | ↓ aggregation of mutant htt exon 1 protein, polyQ-mediated htt protein aggregation, cytotoxicity | [91] | ||
| Vitis vinifera | trans-(-)-ε-Viniferin | STHdhQ7/Q7, STHdhQ111/Q111, Tet-Off PC12 cells, Neuroblastoma N2a cells and Primary cortical neurons | 1 nM, 10nM, 100nM, 1 µM | ↓ cell toxicity, oxidative stress, mitochondrial dysfunction, ↑ mitochondrial genesis, SIRT3-dependent AMPK Activation | [92] | ||
| Withania Somnifera | W. somnifera root extract | 3-NP-induced HD | 100 and 200 mg/kg for 14 days | ↑ body weight, behavioral activities, SOD, catalase, mitochondrial complex (I, II, III) levels, ↓ LPO, nitrite, LDH | [93] | ||
| Vascular Cognitive impairment | Aster ageratoides | Aster ageratoides extract (AAE) | PC 12 cells treated with 50 µM glutamate or 2VO/H surgery in Sprague Dawley rats | 0, 10, 25, and 50 µg/mL or 50 mg/kg b.w | ↓ Memory impairment in vivo, ↓ hippocampal structures, neuronal excitotoxicity in vitro | [94] | |
| Ginkgo biloba L | bilobalide | male Sprague Dawley rats (2-vessel occlusion, 2-VO) | 2, 4 and 8 mg/kg | ↓ Memory impairment, ↓ nuclear condensation, ↑SOD and GSH, ↓ NOS and MDA, TNF-α | [95] | ||
| EGb761 | bilateral common carotid arteries repeated occlusion in rats and ip injection of sodium nitroprusside | 50 mg/kg | ↓ Memory impairment, proliferation of neural stem cells in dentate gyrus and subventricular zone | [96] | |||
| Ginkgo biloba extract | 90 patients, randomized, double-blind, placebo-controlled trial | 60 and 120 mg | ↑ CGI scores, no effect on trans cranial Doppler ultrasound, more adverse reactions in Placebo group. | [97] | |||
| Huperzia serrata | Huperzine | randomized, double-blinded, placebo-controlled study with 78 patients with mild to moderate VaD | 0.1-mg bid | ↑ MMSE, CDR, and ADL scores | [98,99] | ||
| Stroke (causes secondary neurodegeneration) | Grapes and other plants | Resveratrol | Primary cortical neuron cultures with OGD/R | 1, 5, and 20 µmol/L for 24 h | ↑ cell viability, neurite outgrowth and synaptogenesis via Shh signaling pathway, Sirt1 activation | [100] | |
| (MCAO/R) Model | 30 mg/kg for 7 days | ↑ behavior deficits, ↓ infarction volume, ↑ NeuN+ cells, ↓ TUNEL+ cells | [101] | ||||
| Ginkgo biloba | Ginkgolides | NSC line | 20, 40 or 60 mg/L | ↑ cell viability, process length and cell body area, sizes of NSE, GFAP and SOCS2-positive cells | [102] | ||
| mouse model of myocardial I/R injury | 2.5 mL/kg | ↑ cardiac function, ↓ LDH and AST, TWEAK expression, infarction volume | [102] | ||||
| Curcuma longa | Curcumin | Mouse N2a cells hypoxia model | 5, 15, 25, and 35 μmol/L for 24 h | ↑ cell viability, mitochondrial disruption, Bcl2 expression, ↓ Tunnel positive cells, Bax and Caspase-3 expression | [103] | ||
| Rat model of global brain ischemia | 25 and 50 mg/kg | ↑ DA and its metabolites DOPAC and HVANE and 5-HT, cell viability, ↓ COX-2, TNF-α expression | [104] | ||||
| Green tea | EGCG | HBMVECs OGD/R model | 2 μM for 24 h | ↑ cell viability, SOD, migration and tube formation, mRNA expression of VEGF, Bcl2, ↓ apoptosis and autophagy, ROS, LDH, MDA, mRNA expression of Bax and Caspase-3 | [105] | ||
| Rat MCAO model | 20 mg/kg | ↓ infarct volume, TUNEL+ cells, NO, MDA, ↑ Behavioral parameters, SOD and GPx | [106] | ||||
| Scutellaria baicalensis | Baicalin/baicalein | Rat MCAO model | 200 mg/kg, 24 h after reperfusion till 7 days | ↑ behavior deficits, microglia/macrophage M2 markers CD206 and CD 163, ↓infarct volume, microglia/ macrophage M1 markers CD86 and CD 16, MAPK and NF-κB | [107] | ||
| Angelica sinensis | Ligustilide | Rat MCAO model | 7.5, 15 or 30 mg/kg for 3 days | ↑ behavior deficits, ↓ infarct volume, ↑ HSP-70 and MAPK activation | [108] | ||
| Sinomenium acutum | Sinomenine | Mice MCAO model | 10 or 20 mg/kg daily for 3 days | ↑ behavior deficits, ↓ infarct volume, apoptosis, astrocyte and microglial activation, NLRP3 inflammasome, IL-1β, IL-6, IL-18 and TNF-α generation | [109] | ||
| Magnolia officinalis | Honokiol | Rat cerebral ischemia reperfusion model | 0.7–70 µg/kg, 15 min after ischemia | ↓ Cerebral edema, p65 level, NO, TNF- α, RANTES/CCL5 levels | [110] | ||
| Zingiber officinale | Zingerone | Rat MCAO model | 50, 100 mg/kg at 5 h and 12 h after initiation of surgery | ↑ behavior deficits, ↓ infarct volume, LPO, Caspase-3 and -9 Apaf-1, Bax, ↑ GSH and dependent enzymes, Catalase, Bcl-2 | [111] | ||
| Many plants | Perillyl alcohol | Rat MCAO model | 25, 50, 100 mg/kg for 7 days | ↑ behavior deficits, ↓ infarct volume, LPO, TNF- α, IL-1β, IL-6, COX-2, iNOS, NF-κB, ↑ GSH and dependent enzymes, Catalase | [112] | ||
| Piper nigrum | Piperine | Rat MCAO model | 10 mg/kg for 15 days | ↓ infarct volume, ↑ behavior deficits and histopathological findings, ↓ TNF- α, IL-1β, IL-6, COX-2, iNOS, NF-κB | [113] | ||
| Prion Disease | Scutellaria biacalenesis | Baicalein | PrP 106-126-induced-(SH-SY5Y and PrP 106-126 and SK-N-SH) cells | 80 µM | ↓ROS production, ↓ mitochondrial dysfunction, ↓ apoptosis, ↓ JNK signalling | [114] | |
| Grapes and other plants | Resveratrol | PrP 106-126-induced-(SH-SY5Y and PrP 106-126 and SK-N-SH) cells | 2–4 µM | ↑ autophagy, ↓ mitochondrial dysfunction, ↓ apoptosis | [115] | ||
| Cupressaceous plants | Hinokitiol | PrP 106-126-induced SK-N-SH | 8 µM | ↑ HIF-1α, ↑ autophagy, ↑ p62/SQSTM1, ↓ apoptosis | [116] | ||
| Many plants | Rutin | PrP induced-HT22 cells | 10 µg/mL | ↓ ROS and NO, ↓ caspase 3 activity, ↓ caspase 8, FAS, FASL, ↑ BDNF | [117] | ||
| Frontotemporal dementia | Many plants | Nicotine | Grn -/-mice | 0.6 mg/kg P.I daily for 14 days | ↓ CD68, IL-1β, CD11b, ↑ sociability | [118] | |
| Curcuma longa | 250–500 µg/mL | ↓ AChE | [119] | ||||
| Piper nigrum | 250–500 µg/mL | ↓ AChE | [119] | ||||
| Pick’s disease | Curcuma longa | Curcumin | Primary astrocytes from NPC +/+ and NPC -/- mice | 30 µM | ↑ cytosolic Ca2+, ↑ viability | [120] | |
| Many plants | δ-tocopherol | Human fibroblast and baby hamster kidney cells | 40 µM | ↓ cholesterol accumulation, ↓ lysosomal size, ↑ intracellular Ca2+, ↑ Ca2+ deficiency | [121] | ||
| Many plants | Quercetin | Coca-2 cells and male Wistar rats | 100 µM and 5 mg/kg (rats) | ↓Cholesterol uptake | [122] | ||
| Many plants | Luteolin | Coca-2 cells and male Wistar rats | 100 µM and 5 mg/kg (rats) | ↓Cholesterol uptake | [122] | ||
| Spinocerebellar ataxia | Melibiose | 293 cells and SCA17 transgenic mice | 100 nm - 100 µM for 6 days and daily I.P for days (24 mg/kg) | ↓ polyQ aggregration. ↓ ROS, ↑ autophagy, ↓ caspase 3 | [123,124] | ||
| Spinal muscular atrophy | Brucea javanica | Bruceine D | SMA mice and Δ7 mice | 10 to 30 mg/kg i.p once a day for 7 days | Correcting the splice defect in SMN2, ameliorating SMN phenotype defects | [125] | |
| Tripterygium wilfordii | Triptolide | SMA mice, NSC34 and N18TG2 cells | 0.1 mg/kg I.P daily | ↑ SMN, Gemin2 and Gemin3 expression levels, ↑ transcription of SMN, ↑ survival rate and ↓ SMA related defects | [126] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Jahan, S.; Imtiyaz, Z.; Alshahrani, S.; Antar Makeen, H.; Mohammed Alshehri, B.; Kumar, A.; Arafah, A.; Rehman, M.U. Neuroprotection: Targeting Multiple Pathways by Naturally Occurring Phytochemicals. Biomedicines 2020, 8, 284. https://doi.org/10.3390/biomedicines8080284
Khan A, Jahan S, Imtiyaz Z, Alshahrani S, Antar Makeen H, Mohammed Alshehri B, Kumar A, Arafah A, Rehman MU. Neuroprotection: Targeting Multiple Pathways by Naturally Occurring Phytochemicals. Biomedicines. 2020; 8(8):284. https://doi.org/10.3390/biomedicines8080284
Chicago/Turabian StyleKhan, Andleeb, Sadaf Jahan, Zuha Imtiyaz, Saeed Alshahrani, Hafiz Antar Makeen, Bader Mohammed Alshehri, Ajay Kumar, Azher Arafah, and Muneeb U. Rehman. 2020. "Neuroprotection: Targeting Multiple Pathways by Naturally Occurring Phytochemicals" Biomedicines 8, no. 8: 284. https://doi.org/10.3390/biomedicines8080284