Alcohol Exposure Impacts the Composition of HeLa-Derived Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Ethanol Dosing
2.3. Cell Viability by Trypan Blue Exclusion
2.4. EV Purification and Isolation
2.5. EV Analysis by NTA
2.6. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis and Western Blot Analyses
2.7. Dot Blot Analysis
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Statistical Data
3. Results
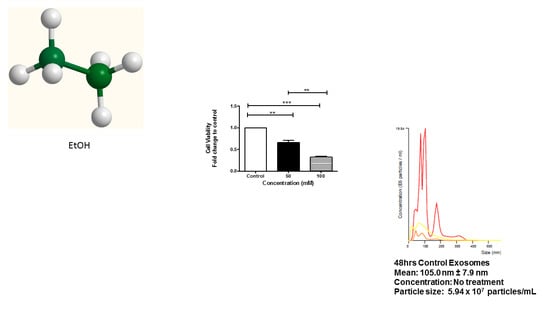
3.1. Cell Viability of HeLa Cells after EtOH Administration
3.2. Characterization of EVs
3.3. Expression of Rab Proteins
3.4. Alcohol Dosing Increases Heat Shock Proteins within EVs
3.5. Alcohol Dosing Alters Apoptotic Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Accessibility
Acknowledgments
Conflicts of Interest
References
- Madsen, B.S.; Jensen, H.L.; van den Brule, A.J.C.; Wohlfahrt, J.; Frisch, M. Risk factors for invasive squamous cell carcinoma of the vulva and vagina—Population-based case–control study in Denmark. Int. J. Cancer 2008, 122, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- De Toro, J.; Herschlik, L.; Waldner, C.; Mongini, C. Emerging roles of exosomes in normal and pathological conditions: New insights for diagnosis and therapeutic applications. Front. Immunol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.M.; Fooladi, A.A.; Nourani, M.R.; Ghanezadeh, F. The role of exosomes in infectious diseases. Inflamm. Allergy Drug Targets 2013, 12, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, J.; Huang, B.; Liu, J.; Chen, X.; Chen, X.M.; Xu, Y.M.; Huang, L.F.; Wang, X.Z. Exosomes: Novel biomarkers for clinical diagnosis. Sci. World J. 2015, 2015, 657086. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; De La Pena, H.; Seifalian, A.M. The application of exosomes as a nanoscale cancer vaccine. Int. J. Nanomed. 2010, 5, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Campanella, C.; Caruso Bavisotto, C.; Logozzi, M.; Marino Gammazza, A.; Mizzoni, D.; Cappello, F.; Fais, S. On the Choice of the Extracellular Vesicles for Therapeutic Purposes. Int. J. Mol. Sci. 2019, 20, 236. [Google Scholar] [CrossRef]
- Zhu, L.; Li, J.; Gong, Y.; Wu, Q.; Tan, S.; Sun, D.; Xu, X.; Zuo, Y.; Zhao, Y.; Wei, Y.Q.; et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol. Cancer 2019, 18, 74. [Google Scholar] [CrossRef]
- Gong, L.; Yan, Q.; Zhang, Y.; Fang, X.; Liu, B.; Guan, X. Cancer cell reprogramming: A promising therapy converting malignancy to benignity. Cancer Commun. 2019, 39, 48. [Google Scholar] [CrossRef]
- Khan, S.; Jutzy, J.M.; Aspe, J.R.; McGregor, D.W.; Neidigh, J.W.; Wall, N.R. Survivin is released from cancer cells via exosomes. Apoptosis Int. J. Program. Cell Death 2011, 16, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Wang, X.; Yu, Q.; Li, S.; Yu, X.; Gong, W. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int. J. Mol. Sci. 2014, 15, 758–773. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, W.; Yang, B.; Tian, H. ATF1 and RAS in exosomes are potential clinical diagnostic markers for cervical cancer. Cell Biochem. Funct. 2017, 35, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.F.; Ma, J.; Huang, L.; Yi, H.Y.; Zhang, Y.M.; Wu, X.G.; Yan, R.M.; Liang, L.; Zhong, M.; Yu, Y.H.; et al. Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene 2019, 38, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Honegger, A.; Schilling, D.; Sultmann, H.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Identification of E6/E7-Dependent MicroRNAs in HPV-Positive Cancer Cells. Methods Mol. Biol. 2018, 1699, 119–134. [Google Scholar] [CrossRef]
- Jin, Y.; Guan, Z.; Wang, X.; Wang, Z.; Zeng, R.; Xu, L.; Cao, P. ALA-PDT promotes HPV-positive cervical cancer cells apoptosis and DCs maturation via miR-34a regulated HMGB1 exosomes secretion. Photodiagnosis Photodyn. Ther. 2018, 24, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Sharma, A.; Bharti, A.C. Upstream Hedgehog signaling components are exported in exosomes of cervical cancer cell lines. Nanomedicine 2018, 13, 2127–2138. [Google Scholar] [CrossRef]
- Lucido, C.T.; Wynja, E.; Madeo, M.; Williamson, C.S.; Schwartz, L.E.; Imblum, B.A.; Drapkin, R.; Vermeer, P.D. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol. Oncol. 2019, 154, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Mata-Rocha, M.; Rodriguez-Hernandez, R.M.; Chavez-Olmos, P.; Garrido, E.; Robles-Vazquez, C.; Aguilar-Ruiz, S.; Torres-Aguilar, H.; Gonzalez-Torres, C.; Gaytan-Cervantes, J.; Mejia-Arangure, J.M.; et al. Presence of HPV DNA in extracellular vesicles from HeLa cells and cervical samples. Enferm. Infecc. Y Microbiol. Clin. 2019. [Google Scholar] [CrossRef]
- Berti, F.C.B.; Salviano-Silva, A.; Beckert, H.C.; de Oliveira, K.B.; Cipolla, G.A.; Malheiros, D. From squamous intraepithelial lesions to cervical cancer: Circulating microRNAs as potential biomarkers in cervical carcinogenesis. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188306. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac Extracellular Vesicles in Normal and Infarcted Heart. Int. J. Mol. Sci. 2016, 17, 63. [Google Scholar] [CrossRef]
- Caruso, S.; Poon, I.K.H. Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.B.; Bell, C.R.; Bibb, K.E.; Gu, L.; Coats, M.T.; Matthews, Q.L. Pathogens and Their Effect on Exosome Biogenesis and Composition. Biomedicines 2018, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Eldh, M.; Ekstrom, K.; Valadi, H.; Sjostrand, M.; Olsson, B.; Jernas, M.; Lotvall, J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE 2010, 5, e15353. [Google Scholar] [CrossRef]
- Corrado, C.; Raimondo, S.; Chiesi, A.; Ciccia, F.; De Leo, G.; Alessandro, R. Exosomes as intercellular signaling organelles involved in health and disease: Basic science and clinical applications. Int. J. Mol. Sci. 2013, 14, 5338–5366. [Google Scholar] [CrossRef] [PubMed]
- Beach, A.; Zhang, H.G.; Ratajczak, M.Z.; Kakar, S.S. Exosomes: An overview of biogenesis, composition and role in ovarian cancer. J. Ovarian Res. 2014, 7, 14. [Google Scholar] [CrossRef]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. CMLS 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Carone, C.; Genedani, S.; Leo, G.; Filaferro, M.; Fuxe, K.; Agnati, L.F. In vitro effects of cocaine on tunneling nanotube formation and extracellular vesicle release in glioblastoma cell cultures. J. Mol. Neurosci. MN 2015, 55, 42–50. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Bala, S.; Kodys, K.; Szabo, G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 2015, 5, 9991. [Google Scholar] [CrossRef] [Green Version]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Gu, L.; Matthews, Q.L. Role of TIM-4 in exosome-dependent entry of HIV-1 into human immune cells. Int. J. Nanomed. 2017, 12, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cheruvanky, A.; Hu, X.; Matsumoto, T.; Hiramatsu, N.; Cho, M.E.; Berger, A.; Leelahavanichkul, A.; Doi, K.; Chawla, L.S.; et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008, 74, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, A.K.; Giebel, B. Exosomes: Small vesicles participating in intercellular communication. Int. J. Biochem. Cell Biol. 2012, 44, 11–15. [Google Scholar] [CrossRef]
- Nakamura, K.; Sawada, K.; Kobayashi, M.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Kimura, T. Role of the Exosome in Ovarian Cancer Progression and Its Potential as a Therapeutic Target. Cancers 2019, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Matejcic, M.; Gunter, M.J.; Ferrari, P. Alcohol metabolism and oesophageal cancer: A systematic review of the evidence. Carcinogenesis 2017, 38, 859–872. [Google Scholar] [CrossRef]
- Campanella, C.; Rappa, F.; Sciume, C.; Marino Gammazza, A.; Barone, R.; Bucchieri, F.; David, S.; Curcuru, G.; Caruso Bavisotto, C.; Pitruzzella, A.; et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar] [CrossRef]
- Sekhon, S.; Massad, L.S.; Hagemann, A.R.; Dick, R.; Leon, A.; Zamorano, A.S.; Thaker, P.H.; McCourt, C.K.; Mutch, D.G.; Powell, M.A.; et al. Patients with endometrial cancer continue to lack understanding of their risks for cancer. Gynecol. Oncol. Rep. 2019, 29, 106–110. [Google Scholar] [CrossRef]
- Choi, Y.J.; Myung, S.K.; Lee, J.H. Light Alcohol Drinking and Risk of Cancer: A Meta-Analysis of Cohort Studies. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2018, 50, 474–487. [Google Scholar] [CrossRef] [Green Version]
- Cappello, F.; Logozzi, M.; Campanella, C.; Bavisotto, C.C.; Marcilla, A.; Properzi, F.; Fais, S. Exosome levels in human body fluids: A tumor marker by themselves? Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 96, 93–98. [Google Scholar] [CrossRef]
- Wyciszkiewicz, A.; Kalinowska-Lyszczarz, A.; Nowakowski, B.; Kazmierczak, K.; Osztynowicz, K.; Michalak, S. Expression of small heat shock proteins in exosomes from patients with gynecologic cancers. Sci. Rep. 2019, 9, 9817. [Google Scholar] [CrossRef]
- Sapan, C.V.; Lundblad, R.L.; Price, N.C. Colorimetric protein assay techniques. Biotechnol. Appl. Biochem. 1999, 29 Pt 2, 99–108. [Google Scholar]
- Blanc, L.; Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Amaya-Farfan, J. Dietary Nutrients and Bioactive Substances Modulate Heat Shock Protein (HSP) Expression: A Review. Nutrients 2018, 10, 683. [Google Scholar] [CrossRef] [PubMed]
- Santos-Junior, V.A.; Lollo, P.C.B.; Cantero, M.A.; Moura, C.S.; Amaya-Farfan, J.; Morato, P.N. Heat Shock Proteins: Protection and Potential Biomarkers for Ischemic Injury of Cardiomyocytes After Surgery. Braz. J. Cardiovasc. Surg. 2018, 33, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Eguchi, T.; Sogawa, C.; Calderwood, S.K.; Futagawa, J.; Kasai, T.; Seno, M.; Okamoto, K.; Sasaki, A.; Kozaki, K.I. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell. Biochem. 2018, 119, 7350–7362. [Google Scholar] [CrossRef]
- Campanella, C.; D’Anneo, A.; Marino Gammazza, A.; Caruso Bavisotto, C.; Barone, R.; Emanuele, S.; Lo Cascio, F.; Mocciaro, E.; Fais, S.; Conway De Macario, E.; et al. The histone deacetylase inhibitor SAHA induces HSP60 nitration and its extracellular release by exosomal vesicles in human lung-derived carcinoma cells. Oncotarget 2016, 7, 28849–28867. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, P.; Hou, Q.; Feng, S.; Liu, L.; Cui, D.; Shi, H.; Fu, Y.; Luo, Y. A novel pan-cancer biomarker plasma heat shock protein 90alpha and its diagnosis determinants in clinic. Cancer Sci. 2019. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 1625–1637. [Google Scholar] [CrossRef] [Green Version]
- Koff, J.L.; Ramachandiran, S.; Bernal-Mizrachi, L. A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci. 2015, 16, 2942–2955. [Google Scholar] [CrossRef]
- Sims, B.; Farrow, A.L.; Williams, S.D.; Bansal, A.; Krendelchtchikov, A.; Matthews, Q.L. Tetraspanin blockage reduces exosome-mediated HIV-1 entry. Arch. Virol. 2018, 163, 1683–1689. [Google Scholar] [CrossRef] [Green Version]
- Petry, K.U. HPV and cervical cancer. Scand. J. Clin. Lab. Investig. 2014, 74, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaliq, S.A.; Shyum Naqvi, S.B.; Fatima, A. Human Pappilomavirus (HPV) induced cancers and prevention by immunization. Pak. J. Pharm. Sci. 2012, 25, 763–772. [Google Scholar] [PubMed]
- Bagby, G.J.; Stoltz, D.A.; Zhang, P.; Kolls, J.K.; Brown, J.; Bohm, R.P., Jr.; Rockar, R.; Purcell, J.; Murphey-Corb, M.; Nelson, S. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol. Clin. Exp. Res. 2003, 27, 495–502. [Google Scholar] [CrossRef]
- Hahn, J.A.; Woolf-King, S.E.; Muyindike, W. Adding fuel to the fire: Alcohol’s effect on the HIV epidemic in Sub-Saharan Africa. Curr. HIV/AIDS Rep. 2011, 8, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ande, A.; Sinha, N.; Rao, P.S.; McArthur, C.P.; Ayuk, L.; Achu, P.N.; Njinda, A.; Kumar, A.; Kumar, S. Enhanced oxidative stress by alcohol use in HIV+ patients: Possible involvement of cytochrome P450 2E1 and antioxidant enzymes. AIDS Res. Ther. 2015, 12, 29. [Google Scholar] [CrossRef]
- Kodidela, S.; Ranjit, S.; Sinha, N.; McArthur, C.; Kumar, A.; Kumar, S. Cytokine profiling of exosomes derived from the plasma of HIV-infected alcohol drinkers and cigarette smokers. PLoS ONE 2018, 13, e0201144. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Leung, C.A.; Douti, L.Y.; Jay, S.M. Ethanol Induces Enhanced Vascularization Bioactivity of Endothelial Cell-Derived Extracellular Vesicles via Regulation of MicroRNAs and Long Non-Coding RNAs. Sci. Rep. 2017, 7, 13794. [Google Scholar] [CrossRef]
- Sims, B.; Gu, L.; Krendelchtchikov, A.; Matthews, Q.L. Neural stem cell-derived exosomes mediate viral entry. Int. J. Nanomed. 2014, 9, 4893–4897. [Google Scholar] [CrossRef] [Green Version]
- Crenshaw, B.J.; Kumar, S.; Bell, C.R.; Jones, L.B.; Williams, S.D.; Saldanha, S.N.; Joshi, S.; Sahu, R.; Sims, B.; Matthews, Q.L. Alcohol Modulates the Biogenesis and Composition of Microglia-Derived Exosomes. Biology 2019, 8, 25. [Google Scholar] [CrossRef]
- Andreu, Z.; Yanez-Mo, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Schulze, R.J.; Rasineni, K.; Weller, S.G.; Schott, M.B.; Schroeder, B.; Casey, C.A.; McNiven, M.A. Ethanol exposure inhibits hepatocyte lipophagy by inactivating the small guanosine triphosphatase Rab7. Hepatol. Commun. 2017, 1, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Vardaki, I.; Sanchez, C.; Fonseca, P.; Olsson, M.; Chioureas, D.; Rassidakis, G.; Ullen, A.; Zhivotovsky, B.; Bjorkholm, M.; Panaretakis, T. Caspase-3-dependent cleavage of Bcl-xL in the stroma exosomes is required for their uptake by hematological malignant cells. Blood 2016, 128, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3052–H3056. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.A.; Kott, K.S.; Poe, A.J.; Kuo, T.; Chen, L.; Ferrara, K.W.; Knowlton, A.A. Cardiac myocyte exosomes: Stability, HSP60, and proteomics. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H954–H965. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef]
- Kumar, S.; Tomar, M.S.; Acharya, A. HSF1-mediated regulation of tumor cell apoptosis: A novel target for cancer therapeutics. Future Oncol. 2013, 9, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, T.; Hirohashi, Y.; Yasuda, K.; Sato, N. Constitutive expression and activation of stress response genes in cancer stem-like cells/tumour initiating cells: Potent targets for cancer stem cell therapy. Int. J. Hyperth. 2013, 29, 436–441. [Google Scholar] [CrossRef]
- Cappello, F.; Marino Gammazza, A.; Palumbo Piccionello, A.; Campanella, C.; Pace, A.; Conway de Macario, E.; Macario, A.J. Hsp60 chaperonopathies and chaperonotherapy: Targets and agents. Expert Opin. Ther. Targets 2014, 18, 185–208. [Google Scholar] [CrossRef]
- Campanella, C.; Bucchieri, F.; Merendino, A.M.; Fucarino, A.; Burgio, G.; Corona, D.F.; Barbieri, G.; David, S.; Farina, F.; Zummo, G.; et al. The odyssey of Hsp60 from tumor cells to other destinations includes plasma membrane-associated stages and Golgi and exosomal protein-trafficking modalities. PLoS ONE 2012, 7, e42008. [Google Scholar] [CrossRef]
- Stetler, R.A.; Gan, Y.; Zhang, W.; Liou, A.K.; Gao, Y.; Cao, G.; Chen, J. Heat shock proteins: Cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. 2010, 92, 184–211. [Google Scholar] [CrossRef] [Green Version]
- Yenari, M.A.; Liu, J.; Zheng, Z.; Vexler, Z.S.; Lee, J.E.; Giffard, R.G. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. N. Y. Acad. Sci. 2005, 1053, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Jin, H.; Steinacker, J.M. Electrical stimulation induced Hsp70 response in C2C12 cells. Exerc. Immunol. Rev. 2010, 16, 86–97. [Google Scholar] [PubMed]
- Bharati, J.; Dangi, S.S.; Chouhan, V.S.; Mishra, S.R.; Bharti, M.K.; Verma, V.; Shankar, O.; Yadav, V.P.; Das, K.; Paul, A.; et al. Expression dynamics of HSP70 during chronic heat stress in Tharparkar cattle. Int. J. Biometeorol. 2017, 61, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Dangi, S.S.; Gupta, M.; Maurya, D.; Yadav, V.P.; Panda, R.P.; Singh, G.; Mohan, N.H.; Bhure, S.K.; Das, B.C.; Bag, S.; et al. Expression profile of HSP genes during different seasons in goats (Capra hircus). Trop. Anim. Health Prod. 2012, 44, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Sogawa, C.; Okusha, Y.; Uchibe, K.; Iinuma, R.; Ono, K.; Nakano, K.; Murakami, J.; Itoh, M.; Arai, K.; et al. Organoids with cancer stem cell-like properties secrete exosomes and HSP90 in a 3D nanoenvironment. PLoS ONE 2018, 13, e0191109. [Google Scholar] [CrossRef]
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 2005, 118, 3631–3638. [Google Scholar] [CrossRef] [Green Version]
- Neckers, L.; Workman, P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 64–76. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Clark, G.M.; Tandon, A.K.; Fuqua, S.A.; Welch, W.J.; McGuire, W.L. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: Prognostic implications. J. Natl. Cancer Inst. 1993, 85, 570–574. [Google Scholar] [CrossRef]
- Carboni, S.; Antonsson, B.; Gaillard, P.; Gotteland, J.P.; Gillon, J.Y.; Vitte, P.A. Control of death receptor and mitochondrial-dependent apoptosis by c-Jun N-terminal kinase in hippocampal CA1 neurones following global transient ischaemia. J. Neurochem. 2005, 92, 1054–1060. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, L.B.; Kumar, S.; Curry, A.J.; Price, J.S.; Krendelchtchikov, A.; Crenshaw, B.J.; Bell, C.R.; Williams, S.D.; Tolliver, T.A.; Saldanha, S.N.; et al. Alcohol Exposure Impacts the Composition of HeLa-Derived Extracellular Vesicles. Biomedicines 2019, 7, 78. https://doi.org/10.3390/biomedicines7040078
Jones LB, Kumar S, Curry AJ, Price JS, Krendelchtchikov A, Crenshaw BJ, Bell CR, Williams SD, Tolliver TA, Saldanha SN, et al. Alcohol Exposure Impacts the Composition of HeLa-Derived Extracellular Vesicles. Biomedicines. 2019; 7(4):78. https://doi.org/10.3390/biomedicines7040078
Chicago/Turabian StyleJones, Leandra B., Sanjay Kumar, Aliyah J. Curry, Jayde S. Price, Alexandre Krendelchtchikov, Brennetta J. Crenshaw, Courtnee’ R. Bell, Sparkle D. Williams, Tambre A. Tolliver, Sabita N. Saldanha, and et al. 2019. "Alcohol Exposure Impacts the Composition of HeLa-Derived Extracellular Vesicles" Biomedicines 7, no. 4: 78. https://doi.org/10.3390/biomedicines7040078