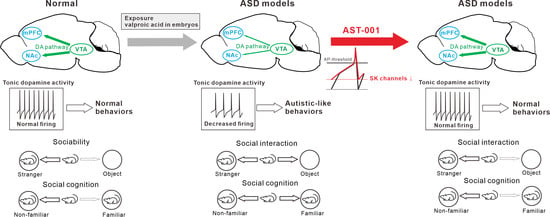
AST-001 Improves Social Deficits and Restores Dopamine Neuron Activity in a Mouse Model of Autism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Induction of VPA-Exposed ASD Mouse Model
2.4. AST-001 Administration
2.5. Behavioral Tests
2.5.1. Three-Chamber Sociability and Social Novelty Test
2.5.2. Elevated Plus Maze (EPM) Test
2.6. Electrophysiology
2.6.1. Midbrain Slice Preparation
2.6.2. Patch Clamp Recording
2.7. Immunostaining
2.8. Quantification and Statistical Analysis
3. Results
3.1. AST-001 Increases Spontaneous Firing in Dopamine Neurons
3.2. AST-001 Alters Afterhyperpolarization through SK Channel Inhibition
3.3. AST-001 Improves Social Interaction in ASD Mouse Models
3.4. AST-001 Improves Anxiety in ASD Mouse Models
3.5. Chronic AST-001 Normalizes Intrinsic Excitability in ASD Mouse Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Pelphrey, K.A.; Shultz, S.; Hudac, C.M.; Wyk, B.C.V. Research Review: Constraining heterogeneity: The social brain and its development in autism spectrum disorder. J. Child Psychol. Psychiatry 2011, 52, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Talantseva, O.I.; Romanova, R.S.; Shurdova, E.M.; Dolgorukova, T.A.; Sologub, P.S.; Titova, O.S.; Kleeva, D.F.; Grigorenko, E.L. The global prevalence of autism spectrum disorder: A three-level meta-analysis. Front. Psychiatry 2023, 14, 1071181. [Google Scholar] [CrossRef] [PubMed]
- A Zaboski, B.; A Storch, E. Comorbid autism spectrum disorder and anxiety disorders: A brief review. Future Neurol. 2018, 13, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.; Ripke, S.; Als, T.D.; Mattheisen, M.; Walters, R.K.; Won, H.; Pallesen, J.; Agerbo, E.; Andreassen, O.A.; Anney, R.; et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 2019, 51, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, C.; Kohls, G.; Troiani, V.; Brodkin, E.S.; Schultz, R.T. The social motivation theory of autism. Trends Cogn. Sci. 2012, 16, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kosillo, P.; Bateup, H.S. Dopaminergic Dysregulation in Syndromic Autism Spectrum Disorders: Insights From Genetic Mouse Models. Front. Neural Circuits 2021, 15, 700968. [Google Scholar] [CrossRef]
- Bariselli, S.; Hörnberg, H.; Prévost-Solié, C.; Musardo, S.; Hatstatt-Burklé, L.; Scheiffele, P.; Bellone, C. Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat. Commun. 2018, 9, 3173. [Google Scholar] [CrossRef]
- Bariselli, S.; Tzanoulinou, S.; Glangetas, C.; Prévost-Solié, C.; Pucci, L.; Viguié, J.; Bezzi, P.; O’Connor, E.C.; Georges, F.; Lüscher, C.; et al. SHANK3 controls maturation of social reward circuits in the VTA. Nat. Neurosci. 2016, 19 Pt A, 926–934. [Google Scholar] [CrossRef]
- Bian, W.-J.; Brewer, C.L.; Kauer, J.A.; de Lecea, L. Adolescent sleep shapes social novelty preference in mice. Nat. Neurosci. 2022, 25, 912–923. [Google Scholar] [CrossRef]
- Nicolini, C.; Fahnestock, M. The valproic acid-induced rodent model of autism. Exp. Neurol. 2018, 299, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-Y.; Liu, F.-C. Pathophysiological Studies of Monoaminergic Neurotransmission Systems in Valproic Acid-Induced Model of Autism Spectrum Disorder. Biomedicines 2022, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Maisterrena, A.; Matas, E.; Mirfendereski, H.; Balbous, A.; Marchand, S.; Jaber, M. The State of the Dopaminergic and Glutamatergic Systems in the Valproic Acid Mouse Model of Autism Spectrum Disorder. Biomolecules 2022, 12, 1691. [Google Scholar] [CrossRef] [PubMed]
- Daghsni, M.; Rima, M.; Fajloun, Z.; Ronjat, M.; Brusés, J.L.; M’Rad, R.; De Waard, M. Autism throughout genetics: Perusal of the implication of ion channels. Brain Behav. 2018, 8, e00978. [Google Scholar] [CrossRef]
- Andrade, A.; Brennecke, A.; Mallat, S.; Brown, J.; Gomez-Rivadeneira, J.; Czepiel, N.; Londrigan, L. Genetic Associations between Voltage-Gated Calcium Channels and Psychiatric Disorders. Int. J. Mol. Sci. 2019, 20, 3537. [Google Scholar] [CrossRef]
- Ping, H.X.; Shepard, P.D. Apamin-sensitive Ca2+-activated K+ channels regulate pacemaker activity in nigral dopamine neurons. NeuroReport 1996, 7, 809–814. [Google Scholar] [CrossRef]
- Kirchner, M.K.; Foehring, R.C.; Armstrong, W.E.; Knowlton, C.; Kutterer, S.; Roeper, J.; Canavier, C.C.; Evans, R.C.; Oster, A.M.; Pissadaki, E.K.; et al. Coupled Oscillator Model of the Dopaminergic Neuron of the Substantia Nigra. J. Neurophysiol. 2000, 83, 3084–3100. [Google Scholar] [CrossRef]
- Garcia-Junco-Clemente, P.; Chow, D.K.; Tring, E.; Lazaro, M.T.; Trachtenberg, J.T.; Golshani, P. Overexpression of calcium-activated potassium channels underlies cortical dysfunction in a model of PTEN -associated autism. Proc. Natl. Acad. Sci. USA 2013, 110, 18297–18302. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, S.-K.; Nam, H.-S.; Cho, J.-S.; Chung, J.-Y. Population Pharmacokinetic Model of AST-001, L-Isomer of Serine, Combining Endogenous Production and Exogenous Administration in Healthy Subjects. Front. Pharmacol. 2022, 13, 891227. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Dunlop, R.A.; Powell, J.T.; Banack, S.A.; Cox, P.A. L-Serine: A Naturally-Occurring Amino Acid with Therapeutic Potential. Neurotox. Res. 2018, 33, 213–221. [Google Scholar] [CrossRef]
- Yamamori, H.; Hashimoto, R.; Fujita, Y.; Numata, S.; Yasuda, Y.; Fujimoto, M.; Ohi, K.; Umeda-Yano, S.; Ito, A.; Ohmori, T.; et al. Changes in plasma d-serine, l-serine, and glycine levels in treatment-resistant schizophrenia before and after clozapine treatment. Neurosci. Lett. 2014, 582, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jégo, P.; Vigneron, P.-A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y.; et al. Impairment of Glycolysis-Derived l-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020, 31, 503–517.e8. [Google Scholar] [CrossRef] [PubMed]
- Krey, I.; von Spiczak, S.; Johannesen, K.M.; Hikel, C.; Kurlemann, G.; Muhle, H.; Beysen, D.; Dietel, T.; Møller, R.S.; Lemke, J.R.; et al. L-Serine Treatment is Associated with Improvements in Behavior, EEG, and Seizure Frequency in Individuals with GRIN-Related Disorders Due to Null Variants. Neurotherapeutics 2022, 19, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Hwang, S.-K.; Park, S.Y.; Kim, M.J.; Jun, D.Y.; Kim, Y.H. l-Serine protects mouse hippocampal neuronal HT22 cells against oxidative stress-mediated mitochondrial damage and apoptotic cell death. Free. Radic. Biol. Med. 2019, 141, 447–460. [Google Scholar] [CrossRef]
- LeClerc, S.; Easley, D. Pharmacological therapies for autism spectrum disorder: A review. P T 2015, 40, 389–397. [Google Scholar]
- Rein, B.; Ma, K.; Yan, Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 2020, 15, 3464–3477. [Google Scholar] [CrossRef]
- Um, K.B.; Hahn, S.; Kim, S.W.; Lee, Y.J.; Birnbaumer, L.; Kim, H.J.; Park, M.K. TRPC3 and NALCN channels drive pacemaking in substantia nigra dopaminergic neurons. eLife 2021, 10, e70920. [Google Scholar] [CrossRef]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Ion Channels and the Electrical Properties of Membranes; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26910/ (accessed on 11 June 2023).
- Imbrici, P.; Camerino, D.C.; Tricarico, D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front. Genet. 2013, 4, 76. [Google Scholar] [CrossRef]
- Mandic-Maravic, V.; Grujicic, R.; Milutinovic, L.; Munjiza-Jovanovic, A.; Pejovic-Milovancevic, M. Dopamine in Autism Spectrum Disorders—Focus on D2/D3 Partial Agonists and Their Possible Use in Treatment. Front. Psychiatry 2022, 12, 787097. [Google Scholar] [CrossRef]
- Mackenzie, B.; Schäfer, M.K.-H.; Erickson, J.D.; Hediger, M.A.; Weihe, E.; Varoqui, H. Functional Properties and Cellular Distribution of the System A Glutamine Transporter SNAT1 Support Specialized Roles in Central Neurons. J. Biol. Chem. 2003, 278, 23720–23730. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Johnson, S.W. Glycine receptor-mediated inhibition of dopamine and non-dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 2001, 919, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Estep, C.M.; Galtieri, D.J.; Zampese, E.; Goldberg, J.A.; Brichta, L.; Greengard, P.; Surmeier, D.J. Transient Activation of GABAB Receptors Suppresses SK Channel Currents in Substantia Nigra Pars Compacta Dopaminergic Neurons. PLoS ONE 2016, 11, e0169044. [Google Scholar] [CrossRef] [PubMed]
- Mabunga, D.F.N.; Gonzales, E.L.T.; Kim, J.-W.; Kim, K.C.; Shin, C.Y. Exploring the Validity of Valproic Acid Animal Model of Autism. Exp. Neurobiol. 2015, 24, 285–300. [Google Scholar] [CrossRef]
- Grace, A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience 1991, 41, 1–24. [Google Scholar] [CrossRef]
- Sharma, S.; Woolfson, L.M.; Hunter, S.C. Maladaptive cognitive appraisals in children with high-functioning autism: Associations with fear, anxiety and theory of mind. Autism 2014, 18, 244–254. [Google Scholar] [CrossRef]
- Farassat, N.; Costa, K.M.; Stojanovic, S.; Albert, S.; Kovacheva, L.; Shin, J.; Egger, R.; Somayaji, M.; Duvarci, S.; Schneider, G.; et al. In vivo functional diversity of midbrain dopamine neurons within identified axonal projections. eLife 2019, 8, e48408. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Um, K.B.; Kwak, S.; Cheon, S.-H.; Kim, J.; Hwang, S.-K. AST-001 Improves Social Deficits and Restores Dopamine Neuron Activity in a Mouse Model of Autism. Biomedicines 2023, 11, 3283. https://doi.org/10.3390/biomedicines11123283
Um KB, Kwak S, Cheon S-H, Kim J, Hwang S-K. AST-001 Improves Social Deficits and Restores Dopamine Neuron Activity in a Mouse Model of Autism. Biomedicines. 2023; 11(12):3283. https://doi.org/10.3390/biomedicines11123283
Chicago/Turabian StyleUm, Ki Bum, Soyoung Kwak, Sun-Ha Cheon, JuHyun Kim, and Su-Kyeong Hwang. 2023. "AST-001 Improves Social Deficits and Restores Dopamine Neuron Activity in a Mouse Model of Autism" Biomedicines 11, no. 12: 3283. https://doi.org/10.3390/biomedicines11123283