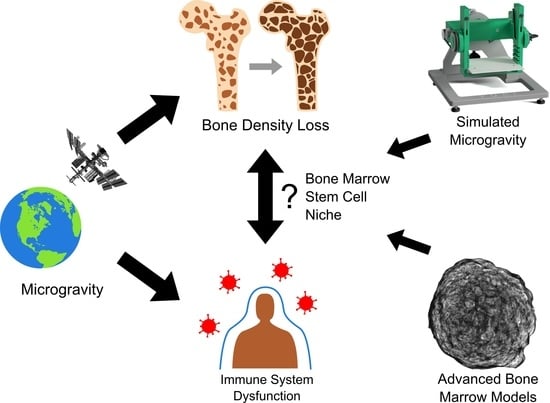
In Vitro Models of Bone Marrow Remodelling and Immune Dysfunction in Space: Present State and Future Directions
Abstract
:1. Hematopoiesis, Innate Immunity, and Spaceflight Conditions
2. Response of the Innate Immune System to Spaceflight Conditions
2.1. Modelling Microgravity
2.2. Response of Innate Immune Cells to Microgravity and Ionizing Radiation
2.2.1. Hematopoietic Stem Cells
2.2.2. Peripheral Blood Mononuclear Cells
2.2.3. Monocytes and Macrophages
2.2.4. Osteocytes
2.2.5. Natural Killer Cells
2.2.6. Granulocytes
2.3. Response to Microgravity of the Innate Immune System in Animal Models
3. Modelling the Bone Marrow Stem Cell Niche
3.1. The Bone Marrow Microenvironment
3.2. D Models of the Bone Marrow Stem Cell Niche
3.2.1. Scaffold-Free 3D Cellular Bone Marrow Models
3.2.2. Scaffold-Based 3D Cellular Bone Marrow Models
3.2.3. Microfluidic and Organ-on-a-Chip Bone Marrow Models
3.3. Adaptation and Application of Bone Marrow Models in Real Microgravity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Cluster of differentiation |
| ECM | Extracellular matrix |
| Gy | Gray |
| HAP | Hydroxyapatite |
| hBM-MSC | Human bone marrow-derived mesenchymal stromal cell |
| HR | Hindlimb unloading followed by a period of recovery |
| HSC | Hematopoietic stem cell |
| HU | Hindlimb unloading |
| ICAM-1 | Intercellular adhesion molecule 1 |
| ISS | International Space Station |
| MSC | Mesenchymal stromal progenitor cell |
| NFκB | Nuclear Factor Kappa B |
| NK | Natural killer cell |
| NLR | Neutrophil-to-lymphocyte ratio |
| PBMC | Peripheral blood mononuclear cell |
| ROS | Reactive oxygen species |
| RPM | Random positioning machine |
| RWV | Rotating wall vessel |
| SCF | Stem Cell Factor |
References
- Institute of Medicine (IOM). Safe Passage: Astronaut Care for Exploration Missions; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-07585-5. [Google Scholar]
- Axpe, E.; Chan, D.; Abegaz, M.F.; Schreurs, A.S.; Alwood, J.S.; Globus, R.K.; Appel, E.A. A human mission to Mars: Predicting the bone mineral density loss of astronauts. PLoS ONE 2020, 15, e0226434. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, J.C.; Scott, G.B.I.; Sutton, J.P. Space radiation: The number one risk to astronaut health beyond low earth orbit. Life 2014, 4, 491–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Domaratskaya, E.I.; Michurina, T.V.; Bueverova, E.I.; Bragina, E.V.; Nikonova, T.A.; Starostin, V.I.; Khrushchov, N.G. Studies on clonogenic hemopoietic cells of vertebrate in space: Problems and perspectives. Adv. Space Res. 2002, 30, 771–776. [Google Scholar] [CrossRef]
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 2009, 106, 1935–1942. [Google Scholar] [CrossRef] [Green Version]
- Blaber, E.; Marçal, H.; Burns, B.P. Bioastronautics: The Influence of Microgravity on Astronaut Health. Astrobiology 2010, 10, 463–473. [Google Scholar] [CrossRef]
- Crucian, B.; Stowe, R.; Mehta, S.; Uchakin, P.; Quiriarte, H.; Pierson, D.; Sams, C. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 2013, 33, 456–465. [Google Scholar] [CrossRef]
- Verhaar, A.P.; Hoekstra, E.; Tjon, A.S.W.; Utomo, W.K.; Deuring, J.J.; Bakker, E.R.M.; Muncan, V.; Peppelenbosch, M.P. Dichotomal effect of space flight-associated microgravity on stress-activated protein kinases in innate immunity. Sci. Rep. 2014, 4, 5468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.D. Spaceflight-induced bone loss: Is there an Osteoporosis Risk? Curr. Osteoporos. Rep. 2013, 11, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sibonga, J.D.; Spector, E.R.; Johnston, S.L.; Tarver, W.J.; Reeves, J.M. Evaluating bone loss in ISS astronauts. Aerosp. Med. Hum. Perform. 2015, 86, A38–A44. [Google Scholar] [CrossRef]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kalafati, L.; Hajishengallis, G.; Chavakis, T. Myelopoiesis in the Context of Innate Immunity. J. Innate Immun. 2018, 10, 365–372. [Google Scholar] [CrossRef]
- Schultze, J.L.; Mass, E.; Schlitzer, A. Emerging Principles in Myelopoiesis at Homeostasis and during Infection and Inflammation. Immunity 2019, 50, 288–301. [Google Scholar] [CrossRef] [Green Version]
- Chou, D.B.; Frismantas, V.; Milton, Y.; David, R.; Pop-Damkov, P.; Ferguson, D.; MacDonald, A.; Vargel Bölükbaşı, Ö.; Joyce, C.E.; Moreira Teixeira, L.S.; et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 2020, 4, 394–406. [Google Scholar] [CrossRef]
- Pecaut, M.J.; Mao, X.W.; Bellinger, D.L.; Jonscher, K.R.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Mohney, R.P.; Gridley, D.S. Is spaceflight-induced immune dysfunction linked to systemic changes in metabolism? PLoS ONE 2017, 12, e0174174. [Google Scholar] [CrossRef] [Green Version]
- Hammond, T.G.; Allen, P.L.; Birdsall, H.H. Effects of space flight on mouse liver versus kidney: Gene pathway analyses. Int. J. Mol. Sci. 2018, 19, 4106. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.R.; Konstantinova, I.; Sonnenfeld, G.; Jennings, R. Changes in the Immune System During and After Spaceflight. Adv. Space Biol. Med. 1997, 6, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Simons, E.R.; Castro, V.A.; Mark Ott, C.; Pierson, D.L. Changes in neutrophil functions in astronauts. Brain. Behav. Immun. 2004, 18, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, I.; Simons, E.R.; Castro, V.A.; Ott, C.M.; Pierson, D.L. Changes in monocyte functions of astronauts. Brain. Behav. Immun. 2005, 19, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Bigley, A.B.; Agha, N.H.; Baker, F.L.; Spielmann, G.; Kunz, H.E.; Mylabathula, P.L.; Rooney, B.V.; Laughlin, M.S.; Mehta, S.K.; Pierson, D.L.; et al. NK cell function is impaired during long-duration spaceflight. J. Appl. Physiol. 2019, 126, 842–853. [Google Scholar] [CrossRef]
- Taylor, G.R.; Neale, L.S.; Dardano, J.R. Immunological analyses of U.S. space shuttle crewmembers. Aviat. Space Environ. Med. 1986, 57, 213–217. [Google Scholar]
- ElGindi, M.; Sapudom, J.; Ibrahim, I.H.; Al-Sayegh, M.; Chen, W.; Garcia-Sabaté, A.; Teo, J.C.M. May the Force Be with You (Or Not): The Immune System under Microgravity. Cells 2021, 10, 1941. [Google Scholar] [CrossRef]
- Häder, D.P.; Braun, M.; Grimm, D.; Hemmersbach, R. Gravireceptors in eukaryotes—a comparison of case studies on the cellular level. NPJ Microgravity 2017, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Borst, A.G.; Van Loon, J.J.W.A. Technology and developments for the random positioning machine, RPM. Microgravity Sci. Technol. 2009, 21, 287–292. [Google Scholar] [CrossRef]
- Wuest, S.L.; Stern, P.; Casartelli, E.; Egli, M. Fluid Dynamics Appearing during Simulated Microgravity Using Random Positioning Machines. PLoS ONE 2017, 12, e0170826. [Google Scholar] [CrossRef]
- Villacampa, A.; Sora, L.; Herranz, R.; Medina, F.J.; Ciska, M. Analysis of graviresponse and biological effects of vertical and horizontal clinorotation in arabidopsis thaliana root tip. Plants 2021, 10, 734. [Google Scholar] [CrossRef]
- Van Loon, J.J.W.A. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Hauslage, J.; Cevik, V.; Hemmersbach, R. Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine). NPJ Microgravity 2017, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brungs, S.; Hauslage, J.; Hemmersbach, R. Validation of Random Positioning Versus Clinorotation Using a Macrophage Model System. Microgravity Sci. Technol. 2019, 31, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; De Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapes, S.; Ortega, M. Understanding Macrophage Differentiation During Space Flight: The Importance of Ground-Based Experiments Before Space Flight. Recent Patents Sp. Technol. 2013, 3, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plett, P.A.; Frankovitz, S.M.; Abonour, R.; Orschell-Traycoff, C.M. Proliferation of human hematopoietic bone marrow cells in simulated microgravity. In Vitro Cell. Dev. Biol. 2001, 37, 73–78. [Google Scholar] [CrossRef]
- Plett, P.A.; Abonour, R.; Frankovitz, S.M.; Orschell, C.M. Impact of modeled microgravity on migration, differentiation, and cell cycle control of primitive human hematopoietic progenitor cells. Exp. Hematol. 2004, 32, 773–781. [Google Scholar] [CrossRef]
- Wang, P.; Tian, H.; Zhang, J.; Qian, J.; Li, L.; Shi, L.; Zhao, Y. Spaceflight/microgravity inhibits the proliferation of hematopoietic stem cells by decreasing Kit-Ras/cAMP-CREB pathway networks as evidenced by RNA-Seq assays. FASEB J. 2019, 33, 5903–5913. [Google Scholar] [CrossRef]
- Puca, A.; Russo, G.; Giordano, A. Properties of mechano-transduction via simulated microgravity and its effects on intracellular trafficking of VEGFR’s. Oncotarget 2012, 3, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.A.; Wiesmann, W.; Kidwell, W.; Cannon, T.; Kerns, L.; Serke, C.; Delaplaine, T.; Pranger, A.; Lee, K.P. Effect of spaceflight on human stem cell hematopoiesis: Suppression of erythropoiesis and myelopoiesis. J. Leukoc. Biol. 1996, 60, 69–76. [Google Scholar] [CrossRef]
- Adamo, L.; Naveiras, O.; Wenzel, P.L.; McKinney-Freeman, S.; Mack, P.J.; Gracia-Sancho, J.; Suchy-Dicey, A.; Yoshimoto, M.; Lensch, M.W.; Yoder, M.C.; et al. Biomechanical forces promote embryonic haematopoiesis. Nature 2009, 459, 1131–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhang, C.; Li, J.; Han, J.; Liu, X.; Yang, H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res. Ther. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.S.; Harley, B.A.C. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv. 2017, 3, e1600455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, D.; Copley, M.; Benz, C.; Dykstra, B.; Bowie, M.; Eaves, C. Regulation of Hematopoietic Stem Cells by the Steel Factor/KIT Signaling Pathway. Clin. Cancer Res. 2008, 14, 1926–1930. [Google Scholar] [CrossRef] [Green Version]
- Tanimura, S.; Takeda, K. ERK signalling as a regulator of cell motility. J. Biochem. 2017, 162, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambacurta, A.; Merlini, G.; Ruggiero, C.; Diedenhofen, G.; Battista, N.; Bari, M.; Balsamo, M.; Piccirillo, S.; Valentini, G.; Mascetti, G.; et al. Human osteogenic differentiation in Space: Proteomic and epigenetic clues to better understand osteoporosis. Sci. Rep. 2019, 9, 8343. [Google Scholar] [CrossRef] [Green Version]
- Pillay, J.; Den Braber, I.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Feiveson, A.H.; Krieger, S.; Brinda, A.M.K.; Von Scheven, G.; Bürkle, A.; Crucian, B.; Wu, H. Synergistic effects of weightlessness, isoproterenol, and radiation on DNA damage response and cytokine production in immune cells. Int. J. Mol. Sci. 2018, 19, 13689. [Google Scholar] [CrossRef] [Green Version]
- Ludtka, C.; Moore, E.; Allen, J.B. The effects of simulated microgravity on macrophage phenotype. Biomedicines 2021, 9, 1205. [Google Scholar] [CrossRef]
- Shi, L.; Tian, H.; Wang, P.; Li, L.; Zhang, Z.; Zhang, J.; Zhao, Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 2021, 18, 1489–1502. [Google Scholar] [CrossRef] [Green Version]
- Thiel, C.S.; Tauber, S.; Lauber, B.; Polzer, J.; Seebacher, C.; Uhl, R.; Neelam, S.; Zhang, Y.; Levine, H.; Ullrich, O. Rapid Morphological and Cytoskeletal Response to Microgravity in Human Primary Macrophages. Int. J. Mol. Sci. 2019, 20, 2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludtka, C.; Silberman, J.; Moore, E.; Allen, J.B. Macrophages in microgravity: The impact of space on immune cells. NPJ Microgravity 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Pergola, C.; Schubert, K.; Pace, S.; Ziereisen, J.; Nikels, F.; Scherer, O.; Hüttel, S.; Zahler, S.; Vollmar, A.M.; Weinigel, C.; et al. Modulation of actin dynamics as potential macrophage subtype-targeting anti-tumour strategy. Sci. Rep. 2017, 7, 41434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adrian, A.; Schoppmann, K.; Sromicki, J.; Brungs, S.; von der Wiesche, M.; Hock, B.; Kolanus, W.; Hemmersbach, R.; Ullrich, O. The oxidative burst reaction in mammalian cells depends on gravity. Cell Commun. Signal. 2013, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Brungs, S.; Kolanus, W.; Hemmersbach, R. Syk phosphorylation–a gravisensitive step in macrophage signalling. Cell Commun. Signal. 2015, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Thiel, C.S.; de Zélicourt, D.; Tauber, S.; Adrian, A.; Franz, M.; Simmet, D.M.; Schoppmann, K.; Hauschild, S.; Krammer, S.; Christen, M.; et al. Rapid adaptation to microgravity in mammalian macrophage cells. Sci. Rep. 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Tauber, S.; Lauber, B.A.; Paulsen, K.; Layer, L.E.; Lehmann, M.; Hauschild, S.; Shepherd, N.R.; Polzer, J.; Segerer, J.; Thiel, C.S.; et al. Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 2017, 12, e0175599. [Google Scholar] [CrossRef] [Green Version]
- Maier, J.A.M. Impact of simulated microgravity on cell cycle control and cytokine release by U937 cells. Int. J. Immunopathol. Pharmacol. 2006, 19, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, K.; Tauber, S.; Goelz, N.; Simmet, D.M.; Engeli, S.; Birlem, M.; Dumrese, C.; Karer, A.; Hunziker, S.; Biskup, J.; et al. Severe disruption of the cytoskeleton and immunologically relevant surface molecules in a human macrophageal cell line in microgravity—Results of an in vitro experiment on board of the Shenzhou-8 space mission. Acta Astronaut. 2014, 94, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, K.; Tauber, S.; Dumrese, C.; Bradacs, G.; Simmet, D.M.; Gölz, N.; Hauschild, S.; Raig, C.; Engeli, S.; Gutewort, A.; et al. Regulation of ICAM-1 in Cells of the Monocyte/Macrophage System in Microgravity. Biomed Res. Int. 2015, 2015, 538786. [Google Scholar] [CrossRef]
- Moser, D.; Sun, S.J.; Li, N.; Biere, K.; Hoerl, M.; Matzel, S.; Feuerecker, M.; Buchheim, J.I.; Strewe, C.; Thiel, C.S.; et al. Cells’ Flow and Immune Cell Priming under alternating g-forces in Parabolic Flight. Sci. Rep. 2019, 9, 11276. [Google Scholar] [CrossRef] [PubMed]
- Pajevic, P.D.; Spatz, J.M.; Garr, J.; Adamson, C.; Misener, L. Osteocyte biology and space flight. Curr. Biotechnol. 2013, 2, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Q.; Yang, H.; Shi, Q. Macrophages and bone inflammation. J. Orthop. Transl. 2017, 10, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell. Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef]
- Ponzetti, M.; Rucci, N. Updates on Osteoimmunology: What’s New on the Cross-Talk Between Bone and Immune System. Front. Endocrinol. 2019, 10, 236. [Google Scholar] [CrossRef]
- Divieti Pajevic, P.; Krause, D.S. Osteocyte regulation of bone and blood. Bone 2019, 119, 13–18. [Google Scholar] [CrossRef]
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front. Immunol. 2020, 11, 58. [Google Scholar] [CrossRef] [Green Version]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Rooney, B.V.; Crucian, B.E.; Pierson, D.L.; Laudenslager, M.L.; Mehta, S.K. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front. Microbiol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Cao, D.; Song, J.; Ling, S.; Niu, S.; Lu, L.; Cui, Z.; Li, Y.; Hao, S.; Zhong, G.; Qi, Z.; et al. Hematopoietic stem cells and lineage cells undergo dynamic alterations under microgravity and recovery conditions. FASEB J. 2019, 33, 6904–6918. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Rykova, M.P.; Grigorieva, V.; Antropova, E.N. Cell interactions in microgravity: Cytotoxic effects of natural killer cells in vitro. J. Gravit. Physiol. 2004, 11, P177–P180. [Google Scholar] [PubMed]
- Buravkova, L.; Romanov, Y.; Rykova, M.; Grigorieva, O.; Merzlikina, N. Cell-to-cell interactions in changed gravity: Ground-based and flight experiments. Acta Astronaut. 2005, 57, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Buravkova, L.B.; Grigor’eva, O.V.; Rykova, M.P.; Grigor’ev, A.I. Cytotoxic activity of natural killer cells in vitro under microgravity. Dokl. Biol. Sci. Proc. Acad. Sci. USSR Biol. Sci. Sect. 2008, 421, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mei, Q.; Huyan, T.; Xie, L.; Che, S.; Yang, H.; Zhang, M.; Huang, Q. Effects of simulated microgravity on primary human NK cells. Astrobiology 2013, 13, 703–714. [Google Scholar] [CrossRef]
- Mylabathula, P.L.; Li, L.; Bigley, A.B.; Markofski, M.M.; Crucian, B.E.; Mehta, S.K.; Pierson, D.L.; Laughlin, M.S.; Rezvani, K.; Simpson, R.J. Simulated microgravity disarms human NK-cells and inhibits anti-tumor cytotoxicity in vitro. Acta Astronaut. 2020, 174, 32–40. [Google Scholar] [CrossRef]
- Shao, D.; Ye, L.; Zhu, B.; Li, Q.; Yang, H.; Shi, J.; Huang, Q.; Zhao, W. Mechanisms of the Effect of Simulated Microgravity on the Cytotoxicity of NK Cells Following the DNA Methylation of NKG2D and the Expression of DAP10. Microgravity Sci. Technol. 2021, 33, 6. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Paul, A.M.; Mhatre, S.D.; Cekanaviciute, E.; Schreurs, A.S.; Tahimic, C.G.T.; Globus, R.K.; Anand, S.; Crucian, B.E.; Bhattacharya, S. Neutrophil-to-Lymphocyte Ratio: A Biomarker to Monitor the Immune Status of Astronauts. Front. Immunol. 2020, 11, 564950. [Google Scholar] [CrossRef]
- Meloni, M.A.; Galleri, G.; Camboni, M.G.; Pippia, P.; Cogoli, A.; Cogoli-Greuter, M. Modeled microgravity affects motility and cytoskeletal structures. J. Gravit. Physiol. 2004, 11, P197–P198. [Google Scholar]
- Blaber, E.A.; Dvorochkin, N.; Torres, M.L.; Yousuf, R.; Burns, B.P.; Globus, R.K.; Almeida, E.A.C. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 2014, 13, 181–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnefoy, J.; Ghislin, S.; Beyrend, J.; Coste, F.; Calcagno, G.; Lartaud, I.; Gauquelin-Koch, G.; Poussier, S.; Frippiat, J.P. Gravitational experimental platform for animal models, a new platform at ESA’s terrestrial facilities to study the effects of micro-and hypergravity on aquatic and rodent animal models. Int. J. Mol. Sci. 2021, 22, 62961. [Google Scholar] [CrossRef] [PubMed]
- Morey, E.R. Spaceflight and Bone Turnover: Correlation with a New Rat Model of Weightlessness. Bioscience 1979, 29, 168–172. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207. [Google Scholar] [CrossRef] [Green Version]
- Pinho, S.; Frenette, P.S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 2019, 20, 303–320. [Google Scholar] [CrossRef]
- Villa, M.M.; Wang, L.; Huang, J.; Rowe, D.W.; Wei, M. Bone tissue engineering with a collagen-hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, N.; Zhang, Y.; Zhao, B.; Zhang, Z.; Zhou, X.; Zhou, Y.; Liu, H.; Zhang, Y.; Liu, J. Enhanced osteogenic proliferation and differentiation of human adipose-derived stem cells on a porous n-HA/PGS-M composite scaffold. Sci. Rep. 2019, 9, 7960. [Google Scholar] [CrossRef]
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593. [Google Scholar] [CrossRef]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Yin, T. The stem cell niches in bone. J. Clin. Investig. 2006, 116, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Di Maggio, N.; Piccinini, E.; Jaworski, M.; Trumpp, A.; Wendt, D.J.; Martin, I. Toward modeling the bone marrow niche using scaffold-based 3D culture systems. Biomaterials 2011, 32, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ehninger, A.; Trumpp, A. The bone marrow stem cell niche grows up: Mesenchymal stem cells and macrophages move in. J. Exp. Med. 2011, 208, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, M.K.; Thiede, M.A.; Haynesworth, S.E.; Bruder, S.P.; Stanton, L. Gerson Human Marrow-Derived Mesenchymal Stem Cells (MSCs) Express Hematopoietic Cytokines and Support Long-Term Hematopoiesis When Differentiated Toward Stromal and Osteogenic Lineages. J. Hematother. Stem Cell Res. 2000, 9, 841–848. [Google Scholar] [CrossRef]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; MacArthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Boulais, P.E.; Frenette, P.S. Making sense of hematopoietic stem cell niches. Blood 2015, 125, 2621–2629. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.N.P.; Calvi, L.M. Concise Review: Current Concepts in Bone Marrow Microenvironmental Regulation of Hematopoietic Stem and Progenitor Cells. Stem Cells 2013, 31, 1044–1050. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, C.; Krause, D.S. “Caught in the net”: The extracellular matrix of the bone marrow in normal hematopoiesis and leukemia. Exp. Hematol. 2020, 89, 13–25. [Google Scholar] [CrossRef]
- Jing, D.; Fonseca, A.V.; Alakel, N.; Fierro, F.A.; Muller, K.; Bornhauser, M.; Ehninger, G.; Corbeil, D.; Ordemann, R. Hematopoietic stem cells in co-culture with mesenchymal stromal cells -modeling the niche compartments in vitro. Haematologica 2010, 95, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Mahadik, B.P.; Harley, B.A.C.C. Engineering the hematopoietic stem cell niche: Frontiers in biomaterial science. Biotechnol. J. 2015, 10, 1529–1545. [Google Scholar] [CrossRef]
- Leisten, I.; Kramann, R.; Ventura Ferreira, M.S.; Bovi, M.; Neuss, S.; Ziegler, P.; Wagner, W.; Knüchel, R.; Schneider, R.K. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- De Barros, A.P.D.N.N.; Takiya, C.M.; Garzoni, L.R.; Leal-Ferreira, M.L.; Dutra, H.S.; Chiarini, L.B.; Meirelles, M.N.; Borojevic, R.; Rossi, M.I.D. Osteoblasts and bone marrow mesenchymal stromal cells control hematopoietic stem cell migration and proliferation in 3D in vitro model. PLoS ONE 2010, 5, e9093. [Google Scholar] [CrossRef] [PubMed]
- Braham, M.V.J.J.; Li Yim, A.S.P.P.; Garcia Mateos, J.; Minnema, M.C.; Dhert, W.J.A.A.; Öner, F.C.; Robin, C.; Alblas, J. A Human Hematopoietic Niche Model Supporting Hematopoietic Stem and Progenitor Cells In Vitro. Adv. Healthc. Mater. 2019, 8, 1801444. [Google Scholar] [CrossRef] [PubMed]
- Fairfield, H.; Falank, C.; Farrell, M.; Vary, C.; Boucher, J.M.; Driscoll, H.; Liaw, L.; Rosen, C.J.; Reagan, M.R. Development of a 3D bone marrow adipose tissue model. Bone 2019, 118, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Di Buduo, C.A.; Laurent, P.A.; Zaninetti, C.; Lordier, L.; Soprano, P.M.; Ntai, A.; Barozzi, S.; La Spada, A.; Biunno, I.; Raslova, H.; et al. Miniaturized 3d bone marrow tissue model to assess response to thrombopoietin-receptor agonists in patients. eLife 2021, 10, e58775. [Google Scholar] [CrossRef]
- Tavakol, D.N.; Bonini, F.; Tratwal, J.; Genta, M.; Brefie-Guth, J.; Braschler, T.; Naveiras, O. Cryogel-based Injectable 3D Microcarrier Co-culture for Support of Hematopoietic Progenitor Niches. Curr. Protoc. 2021, 1, e275. [Google Scholar] [CrossRef]
- Torisawa, Y.S.; Spina, C.S.; Mammoto, T.; Mammoto, A.; Weaver, J.C.; Tat, T.; Collins, J.J.; Ingber, D.E. Bone marrow–on–a–chip replicates hematopoietic niche physiology in vitro. Nat. Methods 2014, 11, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Sieber, S.; Wirth, L.; Cavak, N.; Koenigsmark, M.; Marx, U.; Lauster, R.; Rosowski, M. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med. 2018, 12, 479–489. [Google Scholar] [CrossRef]
- Glaser, D.E.; Curtis, M.B.; Sariano, P.A.; Rollins, Z.A.; Shergill, S.; Anand, A.; Deely, A.M.; Shirure, V.S.; Anderson, L.; Lowen, J.M.; et al. Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials 2021, 280, 121245. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmins, N.; Dietmair, S.; Nielsen, L. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis 2004, 7, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Potekhina, E.; Belousov, V.V.; Lavrentieva, A. Hypoxia Onset in Mesenchymal Stem Cell Spheroids: Monitoring with Hypoxia Reporter Cells. Front. Bioeng. Biotechnol. 2021, 9, 611837. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Duran, E.; Mejia-Cruz, C.C.; Jaramillo-Garcia, L.F.; Leal-Garcia, E.; Barreto-Prieto, A.; Rodriguez-Pardo, V.M. 3d multicellular spheroid for the study of human hematopoietic stem cells: Synergistic effect between oxygen levels, mesenchymal stromal cells and endothelial cells. J. Blood Med. 2021, 12, 517–528. [Google Scholar] [CrossRef]
- Krüger, M.; Pietsch, J.; Bauer, J.; Kopp, S.; Carvalho, D.T.O.; Baatout, S.; Moreels, M.; Melnik, D.; Wehland, M.; Egli, M.; et al. Growth of endothelial cells in space and in simulated microgravity—A comparison on the secretory level. Cell. Physiol. Biochem. 2019, 52, 1039–1060. [Google Scholar] [CrossRef] [Green Version]
- Braccini, A.; Wendt, D.; Jaquiery, C.; Jakob, M.; Heberer, M.; Kenins, L.; Wodnar-Filipowicz, A.; Quarto, R.; Martin, I. Three-Dimensional Perfusion Culture of Human Bone Marrow Cells and Generation of Osteoinductive Grafts. Stem Cells 2005, 23, 1066–1072. [Google Scholar] [CrossRef]
- Fujita, A.; Migita, M.; Ueda, T.; Ogawa, R.; Fukunaga, Y.; Shimada, T. Hematopoiesis in regenerated bone marrow within hydroxyapatite scaffold. Pediatr. Res. 2010, 68, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.C.; Blake, A.L.; Taisto, M.E.; Lehrke, M.J.; Webber, B.R.; Lund, T.C. Dermatopontin in Bone Marrow Extracellular Matrix Regulates Adherence but Is Dispensable for Murine Hematopoietic Cell Maintenance. Stem Cell Rep. 2017, 9, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Lei, X.; Wang, X.; Cai, Z.; Lyu, P.; Zhang, G. Hydroxyapatite/Collagen Three-Dimensional Printed Scaffolds and Their Osteogenic Effects on Human Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Eng. Part A 2019, 25, 1261–1271. [Google Scholar] [CrossRef]
- Park, M.H.; Moon, H.J.; Park, J.H.; Shinde, U.P.; Ko, D.Y.; Jeong, B. PEG-Poly(l-alanine) Thermogel As a 3D scaffold of bone-marrow-derived mesenchymal stem cells. Macromol. Biosci. 2015, 15, 464–472. [Google Scholar] [CrossRef]
- Ventura Ferreira, M.S.; Bergmann, C.; Bodensiek, I.; Peukert, K.; Abert, J.; Kramann, R.; Kachel, P.; Rath, B.; Rütten, S.; Knuchel, R.; et al. An engineered multicomponent bone marrow niche for the recapitulation of hematopoiesis at ectopic transplantation sites. J. Hematol. Oncol. 2016, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rödling, L.; Schwedhelm, I.; Kraus, S.; Bieback, K.; Hansmann, J.; Lee-Thedieck, C. 3D models of the hematopoietic stem cell niche under steady-state and active conditions. Sci. Rep. 2017, 7, 4625. [Google Scholar] [CrossRef] [PubMed]
- Marx-Blümel, L.; Marx, C.; Weise, F.; Frey, J.; Perner, B.; Schlingloff, G.; Lindig, N.; Hampl, J.; Sonnemann, J.; Brauer, D.; et al. Biomimetic reconstruction of the hematopoietic stem cell niche for in vitro amplification of human hematopoietic stem cells. PLoS ONE 2020, 15, e0234638. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, D.N.; Tratwal, J.; Bonini, F.; Genta, M.; Campos, V.; Burch, P.; Hoehnel, S.; Béduer, A.; Alessandrini, M.; Naveiras, O.; et al. Injectable, scalable 3D tissue-engineered model of marrow hematopoiesis. Biomaterials 2020, 232, 119665. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.G.; Barbier, V.; Wadley, R.; Zannettino, A.C.W.; Williams, S.; Lévesque, J.P. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: Serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood 2010, 116, 375–385. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.H.; Jeon, B.; Lim, M.H.; Lee, D.H.; Ye, S.K.; Jeong, S.Y.; Kim, S. 3D cell culture using a clinostat reproduces microgravity-induced skin changes. NPJ Microgravity 2021, 7, 20. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic Alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Sandri, M.; Van Eps, J.; Cabrera, F.J.; Weiner, B.K.; Tampieri, A.; Tasciotti, E. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 2015, 62, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, L.; Jiang, Y.; Wang, C.; Geng, B.; Wang, Y.; Chen, J.; Liu, F.; Qiu, P.; Zhai, G.; et al. Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. FASEB J. 2018, 32, 4444–4458. [Google Scholar] [CrossRef] [Green Version]
- Avitabile, E.; Fusco, L.; Minardi, S.; Orecchioni, M.; Zavan, B.; Yilmazer, A.; Rauner, M.; Pippia, P.; Tasciotti, E.; Delogu, L.G. Bioinspired Scaffold Action Under the Extreme Physiological Conditions of Simulated Space Flights: Osteogenesis Enhancing Under Microgravity. Front. Bioeng. Biotechnol. 2020, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Tabury, K.; Stenuit, H.; Grimm, D.; Baatout, S.; Mironov, V. What can biofabrication do for space and what can space do for biofabrication? Trends Biotechnol. 2022, 40, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendt, D.; Marsano, A.; Jakob, M.; Heberer, M.; Martin, I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnol. Bioeng. 2003, 84, 205–214. [Google Scholar] [CrossRef]
- Angele, P.; Yoo, J.U.; Smith, C.; Mansour, J.; Jepsen, K.J.; Nerlich, M.; Johnstone, B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J. Orthop. Res. 2003, 21, 451–457. [Google Scholar] [CrossRef]
- Gurkan, U.A.; Akkus, O. The mechanical environment of bone marrow: A review. Ann. Biomed. Eng. 2008, 36, 1978–1991. [Google Scholar] [CrossRef]
- De Dong, J.; Gu, Y.Q.; Li, C.M.; Wang, C.R.; Feng, Z.G.; Qiu, R.X.; Chen, B.; Li, J.X.; Zhang, S.W.; Wang, Z.G.; et al. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol. Sin. 2009, 30, 530. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Sakamoto, N.; Song, G.; Sato, M. Low-level shear stress induces human mesenchymal stem cell migration through the SDF-1/CXCR4 axis via MAPK signaling pathways. Stem Cells Dev. 2013, 22, 2384–2393. [Google Scholar] [CrossRef]
- Li, Y.J.; Batra, N.N.; You, L.; Meier, S.C.; Coe, I.A.; Yellowley, C.E.; Jacobs, C.R. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J. Orthop. Res. 2004, 22, 1283–1289. [Google Scholar] [CrossRef]
- Frisch, B.J.; Porter, R.L.; Calvi, L.M. Hematopoietic niche and bone meet. Curr. Opin. Support. Palliat. Care 2008, 2, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Low, L.A.; Giulianotti, M.A. Tissue Chips in Space: Modeling Human Diseases in Microgravity. Pharm. Res. 2020, 37, 8. [Google Scholar] [CrossRef] [PubMed]






| Model Type | Description | Main Characteristics | Advantages | Limitations | Source |
|---|---|---|---|---|---|
| Scaffold-free | 2D Culture | HSC plated on confluent layer of MSC | Multi-layer scaffold-free model with 2 populations of HSC | No complete 3D environment and cell–cell contacts (layers) | [101] |
| Spheroid co-culture | HSC and hBM-MSC co-culture spheroids | Complex structure that resembled trabeculae | Lacks inorganic component | [104] | |
| Cord blood HSC seeded onto non-osteo-induced MSC spheroids | Presence of an endosteal niche | Lacks inorganic component | [114] | ||
| Scaffold-based | Mineral-based | Bioreactor perfusion culture with hBM-MSC on porous disks of hydroxyapatite | Fluid-flow integrated, implantable in mice | Bioreactor form may be unnecessary in microgravity | [118] |
| Hydrogel-based | Co-culture of adipocytes, osteogenic differentiated MSC, endothelial cells and HSC in Matrigel | Proliferation of HSC while maintaining immature hematopoietic progenitor state | Batch-to-batch variability and murine origin of Matrigel, limited downstream applications | [105] | |
| Poly(D,L-lactide-coglycolide) hydrogel with hBM-MSC | Validated in real microgravity | Short term experiment, monoculture | [131] | ||
| Hybrid | 3D printed scaffolds of collagen and hydroxyapatite with hBM-MSC | Closely resembles human trabeculae, ease of synthesis | Not yet validated with co-culture of HSC, mechanics of 3D printing in microgravity | [120] | |
| hBM-MSC in HAP/collagen scaffold that underwent remodelling | Long-term exposure of scaffold to simulated microgravity | Scaffold exhibited compression and collapse of pores, RPM less constant simulation of microgravity | [132] | ||
| Micro-fluidics | In vivo bone synthesis | Development in mice then integration on chip for 7 days | HSC characteristics similar to freshly harvested bone marrow | In vivo bone synthesis phase, relies on cytokines | [109] |
| In vitro bone synthesis | HAP scaffold on which MSC then HSC were seeded | Niche-like environment up to 4 weeks, small footprint | Currently relies on cytokines, frequent medium changes | [110] | |
| Bone marrow-on-a-chip | Both endosteal and perivascular niches maintained for 14 days | More advanced model that includes a mineralized component | Adapting microfluidics for microgravity | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, R.; Pampaloni, F. In Vitro Models of Bone Marrow Remodelling and Immune Dysfunction in Space: Present State and Future Directions. Biomedicines 2022, 10, 766. https://doi.org/10.3390/biomedicines10040766
Sarkar R, Pampaloni F. In Vitro Models of Bone Marrow Remodelling and Immune Dysfunction in Space: Present State and Future Directions. Biomedicines. 2022; 10(4):766. https://doi.org/10.3390/biomedicines10040766
Chicago/Turabian StyleSarkar, Ryan, and Francesco Pampaloni. 2022. "In Vitro Models of Bone Marrow Remodelling and Immune Dysfunction in Space: Present State and Future Directions" Biomedicines 10, no. 4: 766. https://doi.org/10.3390/biomedicines10040766