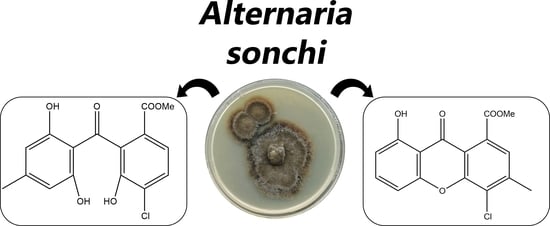
Isolation and Bioactivity of Secondary Metabolites from Solid Culture of the Fungus, Alternaria sonchi
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Production and Purification of Fungal Metabolites
2.3. X-ray Experimental
2.4. Bioassays
2.4.1. Phytotoxic Activity
2.4.2. Antimicrobial Activity
2.4.3. Cytotoxic Activity
2.4.4. Insecticidal Activity
2.4.5. Zootoxicity Assay
2.4.6. Esterase Inhibitory Activity
3. Results
3.1. Structure Elucidation
3.2. Biological Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed Management in 2050: Perspectives on the Future of Weed Science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Hershenhorn, J.; Casella, F.; Vurro, M. Weed Biocontrol with Fungi: Past, Present and Future. Biocontrol Sci. Technol. 2016, 26, 1313–1328. [Google Scholar] [CrossRef]
- Duke, S.O.; Owens, D.K.; Dayan, F.E. Natural Product-Based Chemical Herbicides. In Weed Control: Sustainability, Hazards, and Risks in Cropping Systems Worldwide; Korres, N.E., Burgos, N.R., Duke, S.O., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 153–165. [Google Scholar]
- Graupner, P.R.; Carr, A.; Clancy, E.; Gilbert, J.; Bailey, K.L.; Derby, J.-A.; Gerwick, B.C. The Macrocidins: Novel Cyclic Tetramic Acids with Herbicidal Activity Produced by Phoma macrostoma. J. Nat. Prod. 2003, 66, 1558–1561. [Google Scholar] [CrossRef]
- Bailey, K.L.; Pitt, W.M.; Leggett, F.; Sheedy, C.; Derby, J. Determining the Infection Process of Phoma macrostoma that Leads to Bioherbicidal Activity on Broadleaved Weeds. Biol. Control 2011, 59, 268–276. [Google Scholar] [CrossRef]
- Hubbard, M.; Taylor, W.G.; Bailey, K.L.; Hynes, R.K. The Dominant Modes of Action of Macrocidins, Bioherbicidal Metabolites of Phoma macrostoma, Differ between Susceptible Plant Species. Environ. Exp. Bot. 2016, 132, 80–91. [Google Scholar] [CrossRef]
- Watson, A.K. Microbial Herbicides. In Weed Control: Sustainability, Hazards, and Risks in Cropping Systems Worldwide; Korres, N.E., Burgos, N.R., Duke, S.O., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 133–152. [Google Scholar]
- Skrobek, A.; Boss, D.; Défago, G.; Butt, T.M.; Maurhofer, M. Evaluation of Different Biological Test Systems to Assess the Toxicity of Metabolites from Fungal Biocontrol Agents. Toxicol. Lett. 2006, 161, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, R.E.; Weaver, M.A.; Boyette, C.D.; Abbas, H.K. Bioherbicides: Research and Risks. Toxin Rev. 2007, 26, 313–342. [Google Scholar] [CrossRef]
- Fumagalli, P.; Andolfi, A.; Avolio, F.; Boari, A.; Cimmino, A.; Finizio, A. Ecotoxicological Characterisation of a Mycoherbicide Mixture Isolated from the Fungus Ascochyta caulina. Pest Manag. Sci. 2013, 69, 850–856. [Google Scholar] [CrossRef]
- Yuzikhin, O.; Mitina, G.; Berestetskiy, A. Herbicidal Potential of Stagonolide, a New Phytotoxic Nonenolide from Stagonospora cirsii. J. Agric. Food Chem. 2007, 55, 7707–7711. [Google Scholar] [CrossRef]
- Dalinova, A.; Dubovik, V.R.; Chisty, L.S.; Kochura, D.M.; Ivanov, A.Y.; Smirnov, S.N.; Petrova, M.O.; Zolotarev, A.A.; Evidente, A.; Berestetskiy, A. Stagonolides J and K and Stagochromene A, two New Natural Substituted Nonenolides and a New Disubstituted Chromene-4,5-Dione Isolated from Stagonospora cirsii S-47 Proposed for the Biocontrol of Sonchus arvensis. J. Agric. Food Chem. 2019, 67, 13040–13050. [Google Scholar] [CrossRef]
- Singh nee’ Nigam, P. Production of Bioactive Secondary Metabolites. In Biotechnology for Agro-Industrial Residues Utilisation; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 129–145. [Google Scholar]
- Bannon, J.S. CASST™ Herbicide (Alternaria cassiae): A Case History of a Mycoherbicide. Am. J. Altern. Agric. 1988, 3, 73–76. [Google Scholar] [CrossRef]
- Cook, J.C.; Charudattan, R.; Zimmerman, T.W.; Rosskopf, E.N.; Stall, W.M.; MacDonald, G.E. Effects of Alternaria destruens, Glyphosate, and Ammonium Sulfate Individually and Integrated for Control of Dodder (Cuscuta pentagona). Weed Technol. 2009, 23, 550–555. [Google Scholar] [CrossRef]
- Vieira, B.S.; Barreto, R.W. Liquid Culture Production of Chlamydospores of Lewia Chlamidosporiformans (Ascomycota: Pleosporales), a Mycoherbicide Candidate for Wild Poinsettia. Australas. Plant Pathol. 2010, 39, 154. [Google Scholar] [CrossRef]
- Stubbs, T.L.; Kennedy, A.C. Microbial Weed Control and Microbial Herbicides. In Herbicides—Environmental Impact Studies and Management Approaches; Alvarez-Fernandez, R., Ed.; IntechOpen: London, UK, 2012; pp. 135–166. [Google Scholar]
- Bobylev, M.M.; Bobyleva, L.I.; Strobel, G.A. Synthesis and Bioactivity of Analogs of Maculosin, a Host-Specific Phytotoxin Produced by Alternaria alternata on Spotted Knapweed (Centaurea maculosa). J. Agric. Food Chem. 1996, 44, 3960–3964. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Meng, B.; Wei, R.; Wang, L.; An, C.; Chen, S.; Yang, C.; Qiang, S. An Evaluation of Tenuazonic Acid (Tea) as a Potential Biobased Herbicide in Cotton. Pest Manag. Sci. 2019, 75, 2482–2489. [Google Scholar]
- Pinto, V.E.F.; Patriarca, A. Alternaria Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Humana Press: Totowa, NJ, USA, 2017; pp. 13–32. [Google Scholar]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria Mycotoxins in Food and Feed: An Overview. J. Food Qual. 2017, 2017, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Berestetskiy, A.; Terletskiy, V.M.; Gannibal, P.B.; Kazartsev, I.; Khodorkovskii, M.A. The characteristics of Eurasian isolates of Alternaria sonchi by cultural, molecular, physiological and biochemical characters. Mikol. Fitopatol. 2013, 47, 120–127. [Google Scholar]
- Berestetskiy, A. Gannibal, Ph. Strain of Fungus Alternaria Sonchi G-52 VIZR with Herbicidal Activity against Sowthistle (Sonchus Arvensis L.). Russian Patent RU2701957C1, 28 December 2018. [Google Scholar]
- Evidente, A.; Punzo, B.; Andolfi, A.; Berestetskiy, A.; Motta, A. Alternethanoxins A and B, Polycyclic Ethanones Produced by Alternaria sonchi, Potential Mycoherbicides for Sonchus arvensis Biocontrol. J. Agric. Food Chem. 2009, 57, 6656–6660. [Google Scholar] [CrossRef]
- Berestetskiy, A.; Cimmino, A.; Sofronova, J.; Dalinova, A.; Avolio, F.; Evidente, M.; Chisty, L.; Krivorotov, D.; Evidente, A. Alternethanoxins C–E, Further Polycyclic Ethanones Produced by Alternaria sonchi, a Potential Mycoherbicide for Sonchus arvensis Biocontrol. J. Agric. Food Chem. 2015, 63, 1196–1199. [Google Scholar] [CrossRef]
- Berestetskiy, A.O.; Dalinova, A.A.; Volosatova, N.S. Metabolite Profiles and Biological Activity of Extracts from Alternaria sonchi S-102 Culture Grown by Different Fermentation Methods. Appl. Biochem. Microbiol. 2019, 55, 284–293. [Google Scholar] [CrossRef]
- Cimmino, A.; Pescitelli, G.; Berestetskiy, A.; Dalinova, A.; Krivorotov, D.; Tuzi, A.; Evidente, A. Biological Evaluation and Determination of the Absolute Configuration of Chloromonilicin, a Strong Antimicrobial Metabolite Isolated from Alternaria sonchi. J. Antibiot. 2015, 69, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, S.; Braun, S. 200 and More Basic NMR Experiments: A Practical Course, 1st ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Berestetskiy, A.O.; Yuzikhin, O.S.; Katkova, A.S.; Dobrodumov, A.V.; Sivogrivov, D.E.; Kolombet, L.V. Isolation, Identification, and Characteristics of the Phytotoxin Produced by the Fungus Alternaria cirsinoxia. Appl. Biochem. Microbiol. 2010, 46, 75–79. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Idziorek, T.; Estaquier, J.; De Bels, F.; Ameisen, J.C. YOPRO-1 Permits Cytofluorometric Analysis of Programmed Cell Death (Apoptosis) without Interfering with Cell Viability. J. Immunol. Methods 1995, 185, 249–258. [Google Scholar] [CrossRef]
- Chermenskaya, T.D.; Stepanycheva, E.A.; Shchenikova, A.V.; Savelieva, E.I.; Chakaeva, A.S. Insecticidal Effects of Ungerniase Vertzovii Bulb Extracts Against the Grain Aphid Schizaphis graminum (Rondani). Ind. Crop. Prod. 2012, 36, 122–126. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef]
- Altomare, C.; Pernfuss, B.; Strasser, H. Assessing Potential Cytotoxicity of Biocontrol Microorganisms Using Invertebrate Assays. In Beneficial Microorganisms in Agriculture, Food and the Environment: Safety Assessment and Regulation; Sundh, I., Wilcks, A., Goettel, M., Eds.; CAB International: Wallingford, UK, 2012; pp. 240–255. [Google Scholar]
- Ellman, G.L.; Courtney, D.K.; Andreas, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Yang, Z.P.; Dettbarn, W.D. Prevention of tolerance to the organophosphorus anticholinesterase paraoxon with carboxylesterase inhibitors. Biochem. Pharmacol. 1998, 55, 1419–1426. [Google Scholar] [CrossRef]
- Yamazaki, M.; Okuyama, E. Isolation and Structures of Oxaphenalenone Dimers from Talaromyces bacillosporus. Chem. Pharm. Bull. 1980, 28, 3649–3655. [Google Scholar] [CrossRef] [Green Version]
- Kachi, H.; Sassa, T. Isolation of Moniliphenone, a Key Intermediate in Xanthone Biosynthesis from Monilinia fructicola. Agric. Biol. Chem. 1986, 50, 1669–1671. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, K.; Suzuki, Y.; Sassa, T. Biosynthetic Study of Chloromonilicin, a Growth Self-inhibitor Having a Novel Lactone Ring, from Monilinia fructicola. Agric. Biol. Chem. 1989, 53, 2141–2145. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhang, J.; Song, Z.; Liu, M.; Hu, J.; Hou, C.; Zhu, G.; Jiang, L.; Xia, X.; Quinn, R.J.; et al. Genome- and MS-based Mining of Antibacterial Chlorinated Chromones and Xanthones from the Phytopathogenic Fungus Bipolaris sorokiniana Strain 11134. Appl. Microbiol. Biotechnol. 2019, 103, 5167–5181. [Google Scholar] [CrossRef]
- Hamasaki, T.; Kimura, Y. Isolation and Structures of Four New Metabolites from Aspergillus wentii. Agric. Biol. Chem. 1983, 47, 163–165. [Google Scholar] [CrossRef] [Green Version]
- Sassa, T.; Horiguchi, K.; Suzuki, Y. Chloromonilinic Acids A and B, Novel Catabolites of the Growth Self-inhibitor Chloromonilicin Isolated from Monilinia fructicola. Agric. Biol. Chem. 1989, 53, 1337–1341. [Google Scholar] [CrossRef] [Green Version]
- Masi, M.; Meyer, S.; Clement, S.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Evidente, A. Chloromonilinic Acids C and D, Phytotoxic Tetrasubstituted 3-Chromanonacrylic Acids Isolated from Cochliobolus australiensis with Potential Herbicidal Activity against Buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 2771–2777. [Google Scholar] [CrossRef]
- Holker, J.S.E.; O’Brien, E.; Simpson, T.J. The Structures of Some Metabolites of Penicillium Diversum: A- and Β-Diversonolic Esters. J. Chem. Soc. 1983, 1, 1365–1368. [Google Scholar] [CrossRef]
- Krohn, K.; Kouam, S.F.; Kuigoua, G.M.; Hussain, H.; Cludius-Brandt, S.; Flörke, U.; Kurtan, T.; Pescitelli, G.; Di Bari, L.; Draeger, S.; et al. Xanthones and Oxepino [2, 3-b]chromones from Three Endophytic Fungi. Chem. Eur. J. 2009, 15, 12121–12132. [Google Scholar] [CrossRef]
- Hamasaki, T.; Sato, Y.; Hatsuda, Y. Structure of Sydowinin A, Sydowinin B, and Sydowinol, Metabolites from Aspergillus sydowi. Agric. Biol. Chem. 1975, 39, 2341–2345. [Google Scholar] [CrossRef]
- Shimada, A.; Takahashi, I.; Kawano, T.; Kimurab, Y. Chloroisosulochrin, Chloroisosulochrin Dehydrate, And Pestheic Acid, Plant Growth Regulators, Produced By Pestalotiopsis Theae. Z. Nat. 2001, 56, 797–803. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds—Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2000; pp. 161–243. [Google Scholar]
- Bräse, S.; Gläser, F.; Kramer, C.; Lindner, S.; Linsenmeier, A.M.; Masters, K.-S.; Meister, A.C.; Ruff, B.M.; Zhong, S. The Chemistry of Mycotoxins; Springer: Berlin/Heidelberg, Germany, 2013; pp. 153–205. [Google Scholar]
- Dembitsky, V.M.; Tolstikov, G.A. Natural Clorine-Containing Xanthones. Chem. Sust. Dev. 2004, 12, 13–18. [Google Scholar]
- Qin, C.; Lin, X.; Lu, X.; Wan, J.; Zhou, X.; Liao, S.; Tu, Z.; Xu, S.; Liu, Y. Sesquiterpenoids and Xanthones Derivatives Produced by Sponge-Derived Fungus Stachybotry sp. HH1 ZSDS1F1-2. J. Antibiot. 2014, 68, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, W.; Wang, R.; Du, Y.; Liu, H.; Kong, X.; Li, C. Identification and Bioactivity of Compounds from the Mangrove Endophytic Fungus Alternaria sp. Mar. Drugs 2015, 13, 4492–4504. [Google Scholar] [CrossRef] [Green Version]
- Sassa, T. Overproduction of Moniliphenone, A Benzophenone Biosynthetic Intermediate of Chloromonilicin, by Monilinia fructicola and Its Anthraquinone Precursors. Agric. Biol. Chem. 1991, 55, 95–99. [Google Scholar] [CrossRef]
- Arthan, S.; Tantapakul, C.; Kanokmedhakul, K.; Soytong, K.; Kanokmedhakul, S. A New Xanthone from the Fungus Apiospora montagnei. Nat. Prod. Res. 2017, 31, 1766–1771. [Google Scholar] [CrossRef]
- Narasimhan, S.; Maheshwaran, S.; Abu-Yousef, I.; Majdalawieh, A.; Rethavathi, J.; Das, P.; Poltronieri, P. Anti-Bacterial and Anti-Fungal Activity of Xanthones Obtained via Semi-Synthetic Modification of α-Mangostin from Garcinia mangostana. Molecules 2017, 22, 275. [Google Scholar] [CrossRef] [Green Version]
- Song, X.-Q.; Zhang, X.; Han, Q.-J.; Li, X.-B.; Li, G.; Li, R.-J.; Jiao, Y.; Zhou, J.-H.; Lou, H.-X. Xanthone Derivatives from Aspergillus sydowii, an Endophytic Fungus from the Liverwort Scapania Ciliata, S. Lac and Their Immunosuppressive Activities. Phytochem. Lett. 2013, 6, 318–321. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Gao, Y.-H.; Niu, D.-Y.; Yang, L.-Y.; Gao, X.-M.; Du, G.; Hu, Q.-F. Xanthone Derivatives from the Fermentation Products of an Endophytic Fungus Phomopsis sp. Fitoterapia 2013, 91, 189–193. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Borwornwiriyapan, K.; Phongpaichit, S.; Sakayaroj, J. Benzopyranone, Benzophenone, and Xanthone Derivatives from the Soil Fungus Penicillium citrinum PSU-RSPG95. Tetrahedron Lett. 2014, 55, 1336–1338. [Google Scholar] [CrossRef]
- Ayers, S.; Graf, T.N.; Adcock, A.F.; Kroll, D.J.; Shen, Q.; Swanson, S.M.; Matthew, S.; de Blanco, E.J.C.; Wani, M.C.; Darveaux, B.A.; et al. Cytotoxic Xanthone–Anthraquinone Heterodimers from an Unidentified Fungus of the Order Hypocreales (MSX 17022). J. Antibiot. 2011, 65, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bury, M. Evaluation of the Anticancer Activities of Two Fungal Polycyclic Ethanones, Alternethanoxins A and B, and Two of Their Derivatives. Int. J. Oncol. 2011, 38, 227–232. [Google Scholar] [PubMed]
- Yamada, O.; Kurozumi, A.; Futatsuya, F.; Ito, K.; Ishida, S.; Munakata, K. Studies on Chlorosis-Inducing Activities and Plant Growth Inhibition of Benzophenone Derivatives. Agric. Biol. Chem. 1979, 43, 1467–1471. [Google Scholar] [CrossRef]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria Fungi and Their Bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef]
- Masi, M.; Nocera, P.; Reveglia, P.; Cimmino, A.; Evidente, A. Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies. Molecules 2018, 23, 834. [Google Scholar] [CrossRef] [Green Version]
- De la Cruz Quiroz, R.; Roussos, S.; Hernández, D.; Rodríguez, R.; Castillo, F.; Aguilar, C.N. Challenges and Opportunities of the Bio-Pesticides Production by Solid-State Fermentation: Filamentous Fungi as a Model. Crit. Rev. Biotechnol. 2014, 35, 326–333. [Google Scholar] [CrossRef]
- Lopez-Perez, M.; Rodriguez-Gomez, D.; Loera, O. Production of Conidia of Beauveria bassiana in Solid-State Culture: Current Status and Future Perspectives. Crit. Rev. Biotechnol. 2014, 35, 334–341. [Google Scholar] [CrossRef]
- Sala, A.; Barrena, R.; Artola, A.; Sánchez, A. Current Developments in the Production of Fungal Biological Control Agents by Solid-State Fermentation Using Organic Solid Waste. Crit. Rev. Environ. Sci. Technol. 2019, 49, 655–694. [Google Scholar] [CrossRef]
- Patil, A.S.; Patil, S.R.; Paikrao, H.M. Trichoderma Secondary Metabolites: Their Biochemistry and Possible Role in Disease Management. In Microbial-Mediated Induced Systemic Resistance in Plants; Choudhary, D., Varma, A., Eds.; Springer: Singapore, 2016; pp. 69–102. [Google Scholar]
- Patočka, J. Bioactive Metabolites of Entomopathogenic Fungi Beauveria bassiana. Mil. Med. Sci. Lett. 2016, 85, 80–88. [Google Scholar] [CrossRef]
- Munekata, H. Studies on Some New Metabolic Products of Penicillium II. J. Biochem. 1953, 40, 451–560. [Google Scholar] [CrossRef]
- Healy, P.C.; Hocking, A.; Tran-Dinh, N.; Pitt, J.I.; Shivas, R.G.; Mitchell, J.K.; Kotiw, M.; Davis, R.A. Xanthones from a Microfungus of the Genus Xylaria. Phytochemistry 2004, 65, 2373–2378. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Wang, J.; Zhang, X.; Nong, X.; Xu, X.; Qi, S. Cytotoxic Polyketides from the Deep-Sea-Derived Fungus Engyodontium album DFFSCS021. Mar. Drugs 2014, 12, 5902–5915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Müller, W.E.G.; Mándi, A.; Kurtán, T.; Singab, A.; Lin, W.; Liu, Z.; et al. Xanthones and Sesquiterpene Derivatives from a Marine-Derived Fungus Scopulariopsis sp. Tetrahedron 2016, 72, 2411–2419. [Google Scholar] [CrossRef] [Green Version]
- Moppett, C.E. Revised Structure for Cassiollin: Identity with Pinselin. J. Chem. Soc. D Chem. Commun. 1971, 9, 423. [Google Scholar] [CrossRef]
- Kang, H.-H.; Zhang, H.-B.; Zhong, M.-J.; Ma, L.-Y.; Liu, D.-S.; Liu, W.-Z.; Ren, H. Potential Antiviral Xanthones from a Coastal Saline Soil Fungus Aspergillus iizukae. Mar. Drugs 2018, 16, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wang, R.; Xu, G.; Ding, Z.; Zhu, X.; Li, E.; Liu, L. Two New Polyketides from the Ascomycete Fungus Leptosphaeria sp. J. Antibiot. 2017, 70, 743–746. [Google Scholar] [CrossRef] [PubMed]




| Position (in 1 and 5) | Literature Assignment (5a) [24] | 1 | 5 | ||
|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | ||
| 1 | 1 | 124.0 | - | 148.8 | |
| 2 | 5 | 7.25 1H, s | 124.4 | - | 155.7 |
| 3 | 8 | 144.7 | 7.40 1H, d (9.0) | 125.4 | |
| 4 | 9 | 131.1 | 7.45 1H, d (9.0) | 121.7 | |
| 4a | 6a | 151.9 | - | 150.5 | |
| 4b | 4 | 155.7 | - | 161.2 | |
| 5 | 6 | 6.88 1H, d (8.0) | 111.5 | 6.64 1H, s | 111.3 |
| 6 | 10 | 7.66 1H, t (8.0) | 137.3 | - | 151.9 |
| 7 | 3 | 7.06 1H, d (8.0) | 107.2 | 6.75 1H, s | 107.2 |
| 8 | 10a | 161.7 | - | 155.7 | |
| 8a | 2 | 108.5 | - | 114.7 | |
| 9 | MeCO | 180.5 | - | 180.3 | |
| 9a | 10b | 116.7 | - | 118.5 | |
| 10 | 7 | 169.0 | - | 168.9 | |
| 11 | OMe | 4.04 3H, s | 53.2 | 4.03 3H, s | 53.1 |
| 12 | MeCO | 2.46 3H, s | 20.9 | 2.45 3H, s | 22.6 |
| 8-OH | 12.10 s | 12.23 s | |||
| Position (in 2 and 9) | Literature Assignment (9a) [24] | 2 | 9 | ||
|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | ||
| 1 | 10a | 132.3 | 130.3 | ||
| 2 | 8 | 127.5 | 128.8 | ||
| 3 | 9 | 7.56 1H, d (8.5) | 122.7 | 7.48 1H, d (7.5) | 122.1 |
| 4 | 10 | 7.43 1H, d (8.5) | 128.9 | 7.36 1H, t (7.5) | 130.9 |
| 5 | 2 | 125.2 | 7.15 1H, d (7.5) | 121.4 | |
| 6 | 6a | 147.8 | 153.6 | ||
| 7 | COMe | 197.0 | 199.0 | ||
| 8 | 10b | 109.1 | 107.5 | ||
| 9 | 5 | 160.5 | 160.2 | ||
| 10 | 3 | 6.23 2H, s | 109.3 | 6.24 2H, s | 109.4 |
| 11 | 1 | 148.9 | 148.8 | ||
| 12 | 6 | 6.23 2H, s | 109.3 | 6.24 2H, s | 109.4 |
| 13 | 4 | 160.5 | 160.2 | ||
| 14 | 7 | 166.0 | 167.3 | ||
| 15 | OMe | 3.76 3H, s | 52.6 | 3.65 3H, s | 52.5 |
| 16 | COMe | 2.26 3H, s | 22.1 | 2.25 3H, s | 22.1 |
| Compound | Phytotoxic | Antimicrobial 3 | Insecticidal 4 | |||
|---|---|---|---|---|---|---|
| Sonchus arvensis1 | Elytrigia repens2 | Bacillus subtilis | Escherichia coli | Candida tropicalis | Wheat Aphid | |
| 1 | 0 | 0 | NA | NA | NA | 43.8 ± 12.1 |
| 2 | 2.4 ± 0.2 | 1.1 ± 0.3 | 10 | NA | 50 | nt |
| 3 | 1.5 ± 0.3 | 1.0 ± 0 | NA | NA | NA | 58.8 ± 17.8 |
| 4 | 0 | 0 | <5 | 100 | <5 | 80.0 ± 8.5 |
| 5 | 0 | 0.5 ± 0 | NA | NA | NA | 60.0 ± 4.8 |
| 6 | 0 | 0 | NA | NA | NA | 41.3 ± 8.7 |
| 7 | 0 | 0 | NA | NA | NA | 11.3 ± 6.5 |
| 8 | 0.9 ± 0.2 | 0 | <0.5 | <0.5 | 1 | nt |
| 9 | 1.7 ± 0.2 | 1.2 ± 0.4 | 20 | 20 | 100 | 42.5 ± 23.6 |
| 10 | 1.6 ± 0.5 | 2.0 ± 0.4 | 100 | NA | NA | 73.8 ± 9.1 |
| 11 | 0.7 ± 0.2 | 2.0 ± 0 | 50 | NA | NA | NA |
| 12 | 0.6 ± 0.2 | 1.7 ± 0.3 | 100 | NA | NA | 41.3 ± 12.6 |
| 13 | 0 | 0 | 50 | NA | 20 | 14.5 ± 9.4 |
| Compound | Anti-CE Activity, % 1 | Anti-BCE Activity, % 1 | CE/BCE Activity |
|---|---|---|---|
| 1 | 61.3 | 89.3 | 0.69 |
| 2 | 14.1 | 83.8 | 0.17 |
| 3 | 28.7 | 80.6 | 0.36 |
| 4 | 65.7 | 91.5 | 0.72 |
| 6 | 2.9 | 62.6 | 0.05 |
| 7 | 55.4 | 80.6 | 0.69 |
| 9 | 68.6 | 55.1 | 1.24 |
| 13 | 91.3 | 96.7 | 0.94 |
| Compound | IC50, µM | |
|---|---|---|
| U937 | K562 | |
| 1 | NA | NA |
| 2 | >100 | >100 |
| 3 | >100 | >100 |
| 4 | 72 | >100 |
| 5 | NA | >100 |
| 6 | 90 | >100 |
| 7 | NA | NA |
| 8 | nt | nt |
| 9 | NA | NA |
| 10 | >100 | NA |
| 11 | NA | NA |
| 12 | >100 | >100 |
| 13 | >100 | >100 |
| Etoposide | 0.85 | 83 |
| Compound | Yield from Solid Culture of A. sonchi, mg/kg | Known Producers/Yield | Biological Activity |
|---|---|---|---|
| methyl 8-dihydroxy-3-methyl-4-chloro-9-oxo-9H-xanthene-1-carboxylate (1) | 6 | – * | Weak insecticidal * |
| 5-chloromoniliphenone (2) | 7 | – * | Phytotoxic *, cytotoxic *, antiesterase * |
| 4-chloropinselin (3) | 1879 | Monilinia fructicola [40]/15 mg/L; Bipolaris sorokiniana [42]/2 mg/kg; | Insecticidal * |
| methyl 3,8-dihydroxy-6-methyl-4-chloro-9-oxo-9H-xanthene-1-carboxylate (4) | 29 | B. sorokiniana [42]/1 mg/kg; | Antimicrobial [42], insecticidal *, cytotoxic * |
| pinselin (5) | 223 | Penicillium amarum [71]/ND *; Aspergillus sydowii [58]/4.4 mg/kg; Phomopsis sp. [59]/<1 mg/L; Xylaria sp. [72]/ND; P. citrinum [60]/<1 mg/L; Engyodontium album [73]/3.3 mg/kg; Scopulariopsis sp. [74]/1.8 mg/kg; Cassia occidentalis [75]/ND; | Immunosuppressive [58], cytotoxic [59], insecticidal * |
| methyl 3,8-dihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (6) | 56 | Microsphaeropsis sp. [47]/<1 mg/L; Scopulariopsis sp. [74]/2.5 mg/kg; Aspergillus iizukae [76]/<1 mg/L; | antiviral [76];antiesterase * |
| methyl 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (7) | 46 | M. fructicola [40]/ND; A. sydowii [58]/2.3 mg/kg; P. citrinum [60]/<1 mg/L; | immunosuppressive [58] |
| chloromonilicin (8) | 11 | M. fructicola [40]/74 mg/L; A. sonchi [27]/ND; Cochliobolus australiensis [45]/2.23 mg/L; | antimicrobial [40], antifungal [27] |
| moniliphenone (9) | 154 | M. fructicola [40]/87.4 mg/L; Hypocreales MSX 17022 [61]/112 mg/kg; A. sydowii [58]/6.3 mg/kg; P. citrinum [60]/<1 mg/L; Leptosphaeria sp. [77]/26 mg/kg; | cytotoxic [61] |
| chloromonilinic acid B (10) | 10 | M. fructicola [44]/8 mg/L; B. sorokiniana [42]/<1 mg/L; C. australiensis [45]/16.8 mg/L; | phytotoxic [45], insecticidal * |
| chloromonilinic acid C (12) | 7 | C. australiensis [45]/3.0 mg/L; | phytotoxic [45], cytotoxic * |
| chloromonilinic acid D (11) | 3 | C. australiensis [45]/6.2 mg/L; | phytotoxic [45] |
| α- and β-diversolonic esters (13) | 12 | P. diversum [46]/2.4 mg/L; P. citrinum [60]/<1 mg/L | cytotoxic [61] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalinova, A.; Chisty, L.; Kochura, D.; Garnyuk, V.; Petrova, M.; Prokofieva, D.; Yurchenko, A.; Dubovik, V.; Ivanov, A.; Smirnov, S.; et al. Isolation and Bioactivity of Secondary Metabolites from Solid Culture of the Fungus, Alternaria sonchi. Biomolecules 2020, 10, 81. https://doi.org/10.3390/biom10010081
Dalinova A, Chisty L, Kochura D, Garnyuk V, Petrova M, Prokofieva D, Yurchenko A, Dubovik V, Ivanov A, Smirnov S, et al. Isolation and Bioactivity of Secondary Metabolites from Solid Culture of the Fungus, Alternaria sonchi. Biomolecules. 2020; 10(1):81. https://doi.org/10.3390/biom10010081
Chicago/Turabian StyleDalinova, Anna, Leonid Chisty, Dmitry Kochura, Varvara Garnyuk, Maria Petrova, Darya Prokofieva, Anton Yurchenko, Vsevolod Dubovik, Alexander Ivanov, Sergey Smirnov, and et al. 2020. "Isolation and Bioactivity of Secondary Metabolites from Solid Culture of the Fungus, Alternaria sonchi" Biomolecules 10, no. 1: 81. https://doi.org/10.3390/biom10010081