Enhancing the Drag Reduction Phenomenon within a Rotating Disk Apparatus Using Polymer-Surfactant Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus Description
2.3. TEM
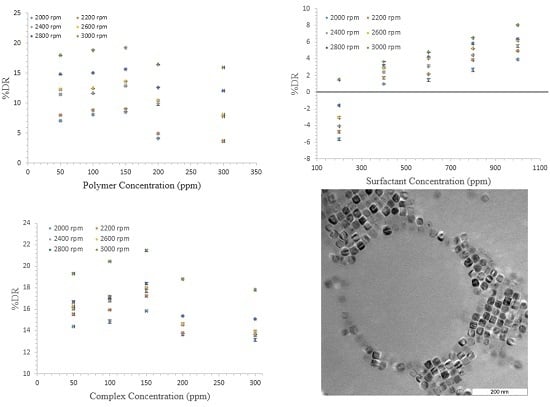
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abubakar, A.; Al-Wahaibi, T.; Al-Wahaibi, Y.; Al-Hashmi, A.R.; Al-Ajmi, A. Roles of drag reducing polymers in single- and multi-phase flows. Chem. Eng. Res. Des. 2014, 92, 2153–2181. [Google Scholar] [CrossRef]
- Choi, H.J.; Jhon, M.S. Polymer-induced turbulent drag reduction. Ind. Eng. Chem. Res. 1996, 35, 2993–2998. [Google Scholar] [CrossRef]
- Kim, C.A.; Jo, D.S.; Choi, H.J.; Kim, C.B.; Jhon, M.S. A high-precision rotating disk apparatus for drag reduction characterization. Polym. Test. 2000, 20, 43–48. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, C.A.; Sung, J.H.; Kim, C.B.; Chun, W.; Jhon, M.S. Universal drag reduction characteristics of saline water-soluble poly(ethylene oxide) in a rotating disk apparatus. Colloid Polym. Sci. 2000, 278, 701–705. [Google Scholar] [CrossRef]
- Lim, S.T.; Hong, C.H.; Choi, H.J.; Lai, P.; Chan, C.K. Polymer turbulent drag reduction near the theta point. Europhys. Lett. 2007, 80, 65–78. [Google Scholar] [CrossRef]
- Sung, J.H.; Lim, S.T.; Kim, C.A.; Chung, H.; Choi, H.J. Mechanical degradation kinetics of poly(ethylene oxide) in a turbulent flow. Korea Aust. Rheol. J. 2004, 16, 57–62. [Google Scholar]
- Kim, N.; Kim, S.; Hoon, S.; Chen, K.; Chun, W. Measurement of drag reduction in polymer added turbulent flow. Int. Commun. Heat Mass Transf. 2009, 36, 1014–1019. [Google Scholar] [CrossRef]
- Yang, K.S.; Choi, H.J.; Kim, C.B.; Jhon, M.S. A study of drag reduction by polymer additives in rotating disk geometry. Korean J. Rheol. 1991, 3, 76–85. [Google Scholar]
- Liberatore, M.W.; Baik, S.; Mchugh, A.J.; Hanratty, T.J. Turbulent drag reduction of polyacrylamide solutions: Effect of degradation on molecular weight distribution. J. Non Newton. Fluid Mech. 2004, 123, 175–183. [Google Scholar] [CrossRef]
- Rao, T.P.; Prasad, P.R.; Sagar, K.S.; Sujatha, V. Effect of polyacrylamide on drag reduction for flow through annular conduits. Int. J. Futur. Sci. Eng. Technol. 2013, 2, 250–260. [Google Scholar]
- Shanshool, J.; Haider, M.T. Effect of molecular weight on turbulent drag reduction with polyisobutylene. In Proceedings of the First Regional Conference of Engineering and Sceince, Mexico City, Mexico, 27–29 April 2008; pp. 52–59.
- Mowla, D.; Naderi, A. Experimental study of drag reduction by a polymeric additive in slug two-phase flow of crude oil and air in horizontal pipes. Chem. Eng. Sci. 2006, 61, 1549–1554. [Google Scholar] [CrossRef]
- Lee, K.; Kim, C.A.; Lim, S.T.; Kwon, D.H.; Choi, H.J.; Jhon, M.S. Mechanical degradation of polyisobutylene under turbulent flow. Colloid Polym. Sci. 2002, 280, 779–782. [Google Scholar] [CrossRef]
- Lee, K.H.; Zhang, K.; Choi, H.J. Time dependence of turbulent drag reduction efficiency of polyisobutylene in kerosene. J. Ind. Eng. Chem. 2010, 16, 499–502. [Google Scholar] [CrossRef]
- Escudier, M.P.; Presti, F.; Smith, S. Drag reduction in the turbulent pipe flow of polymers. J. Nonnewton. Fluid Mech. 1999, 81, 197–213. [Google Scholar] [CrossRef]
- Tian, M.; Fang, B.; Jin, L.; Lu, Y.; Qiu, X.; Jin, H.; Li, K. Rheological and drag reduction properties of hydroxypropyl xanthan gum solutions. Chin. J. Chem. Eng. 2015. [Google Scholar] [CrossRef]
- Wyatt, N.B.; Gunther, C.M.; Liberatore, M.W. Drag reduction effectiveness of dilute and entangled xanthan in turbulent pipe flow. J. Nonnewton. Fluid Mech. 2011, 166, 25–31. [Google Scholar] [CrossRef]
- Hong, C.H.; Zhang, K.; Choi, H.J.; Yoon, S.M. Mechanical degradation of polysaccharide guar gum under turbulent flow. J. Ind. Eng. Chem. 2010, 16, 178–180. [Google Scholar] [CrossRef]
- Kim, C.A.; Lim, S.T.; Choi, H.J.; Sohn, J.I.; Jhon, M.S. Characterization of drag reducing guar gum in a rotating disk flow. J. Appl. Polym. Sci. 2002, 83, 2938–2944. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, C.A.; Sohn, J.; Jhon, M.S. An exponential decay function for polymer degradation in turbulent drag reduction. Polym. Degrad. Stab. 2000, 69, 341–346. [Google Scholar] [CrossRef]
- Myska, J.; Mik, V. Degradation of surfactant solutions by age and by a flow singularity. Chem. Eng. Process. Process. Intensif. 2004, 43, 1495–1501. [Google Scholar] [CrossRef]
- Li, F.; Kawaguchi, Y.; Yu, B.; Wei, J.; Hishida, K. Experimental study of drag-reduction mechanism for a dilute surfactant solution flow. Int. J. Heat Mass Transf. 2008, 51, 835–843. [Google Scholar] [CrossRef]
- Inaba, H.; Aly, W.I.A.; Haruki, N.; Horibe, A. Flow and heat transfer characteristics of drag reducing surfactant solution in a helically coiled pipe. Heat Mass Transf. 2005, 41, 940–952. [Google Scholar] [CrossRef]
- Mohsenipour, A.A.; Pal, R.; Prajapati, K. Effect of cationic surfactant addition on the drag reduction behaviour of anionic polymer solutions. Can. J. Chem. Eng. 2013, 91, 181–189. [Google Scholar] [CrossRef]
- Kwak, J.C. Polymer-Surfactant Systems; Marcel Dekker Inc.: New York, NY, USA, 1998. [Google Scholar]
- Bakshi, M.S.; Kaur, R.; Kaur, I.; Mahajan, R.K.; Sehgal, P.; Doe, H. Unlike surfactant-polymer interactions of sodium dodecyl sulfate and sodium dodecylbenzene sulfonate with water-soluble polymers. Colloid Polym. Sci. 2003, 281, 716–726. [Google Scholar] [CrossRef]
- Spyropoulos, F.; Ding, P.; Frith, W.J.; Norton, I.T.; Wolf, B.; Pacek, A.W. Interfacial tension in aqueous biopolymer-surfactant mixtures. J. Colloid Interface Sci. 2008, 317, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Goddard, E.D. Polymer-surfactant interaction part II. Polymer and surfactant of opposite charge. Colloids Surf. 1986, 19, 301–329. [Google Scholar] [CrossRef]
- Wei, Y.C.; Hudson, S.M. The interaction between polyelectrolytes and surfactants of opposite charge. J. Macromol. Sci. Part C 1995, 35, 15–45. [Google Scholar] [CrossRef]
- Matras, Z.; Kopiczak, B. Intensification of drag reduction effect by simultaneous addition of surfactant and high molecular polymer into the solvent. Chem. Eng. Res. Des. 2015, 96, 35–42. [Google Scholar] [CrossRef]
- Matras, Z.; Malcher, T.; Gzyl-malcher, B. The influence of polymer-surfactant aggregates on drag reduction. Thin Solid Films 2008, 516, 8848–8851. [Google Scholar] [CrossRef]
- Abdulbari, H.; Faraj, J.G.E.; Mahmood, W. Energy dissipation reduction using similarly-charged polymer-surfactant complex. Adv. Appl. Fluid Mech. 2015, 18, 113. [Google Scholar] [CrossRef]
- Bari, H.A.; Yousif, Z.; Akindoyo, E.O. Enhancement of additives polymeric drag resistance to degradation. J. Purity Util. React. Environ. 2015, 4, 48–55. [Google Scholar]
- Kim, J.T.; Kim, C.A.; Zhang, K.; Jang, C.H.; Choi, H.J. Effect of polymer-surfactant interaction on its turbulent drag reduction. Colloids Surf. A Physicochem. Eng. Asp. 2011, 391, 125–129. [Google Scholar] [CrossRef]
- Bari, H.A.; Faraj, E. Studying the interaction between a new mixture in enhancing drag reduction efficiency. Int. J. Chem. Eng. Appl. 2015, 6, 277–280. [Google Scholar]
- Sohn, J.I.; Kim, C.A.; Choi, H.J.; Jhon, M.S. Drag-reduction effectiveness of xanthan gum in a rotating disk apparatus. Carbohydr. Polym. 2001, 45, 61–68. [Google Scholar] [CrossRef]
- Sung, J.H.; Kim, C.A.; Choi, H.J.; Hur, B.K.; Kim, J.G.; Jhon, M.S. Turbulent drag reduction efficiency and mechanical degradation of poly (acrylamide). J. Macromol. Sci. Part B 2004, 43, 507–518. [Google Scholar] [CrossRef]
- Akindoyo, E.O.; Abdulbari, H.A.; Yousif, Z. A dual mechanism of the drag reduction by rigid polymers and cationic surfactant: Complex and nanofluids of xanthan gum and hexadecyl trimethyl ammonium chloride. Int. J. Res. Eng. Technol. 2015, 4, 84–93. [Google Scholar]
- Held, P. Rapid Critical Micelle Concentration (CMC) Determination Using Fluorescence Polarization. Available online: http://www.biotek.com/resources/articles/cmc_determination_using_fluorescence_polarization.html (accessed on 18 September 2014).
- Bari, H.A.A.; Yousif, Z.; Yaacob, Z.B.; Oluwasogaakindoyo, E. Effect of SDBS on the drag reduction characteristics of polyacrylamide in a rotating disk apparatus. Int. J. Basic Appl. Sci. 2015, 4, 326–332. [Google Scholar] [CrossRef]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashed, M.K.; Mohd Salleh, M.A.; Abdulbari, H.A.; Ismail, M.H.S. Enhancing the Drag Reduction Phenomenon within a Rotating Disk Apparatus Using Polymer-Surfactant Additives. Appl. Sci. 2016, 6, 355. https://doi.org/10.3390/app6120355
Rashed MK, Mohd Salleh MA, Abdulbari HA, Ismail MHS. Enhancing the Drag Reduction Phenomenon within a Rotating Disk Apparatus Using Polymer-Surfactant Additives. Applied Sciences. 2016; 6(12):355. https://doi.org/10.3390/app6120355
Chicago/Turabian StyleRashed, Musaab K., Mohamad Amran Mohd Salleh, Hayder A. Abdulbari, and Mohd Halim Shah Ismail. 2016. "Enhancing the Drag Reduction Phenomenon within a Rotating Disk Apparatus Using Polymer-Surfactant Additives" Applied Sciences 6, no. 12: 355. https://doi.org/10.3390/app6120355