The Skin-Whitening Effects of Ectoine via the Suppression of α-MSH-Stimulated Melanogenesis and the Activation of Antioxidant Nrf2 Pathways in UVA-Irradiated Keratinocytes
Abstract
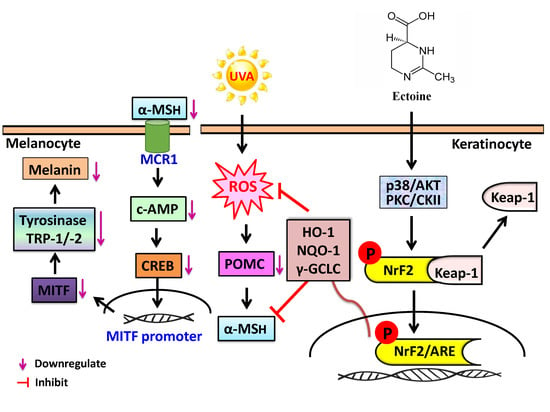
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture
2.3. Cell Treatments and UVA-Irradiation
2.4. Cell Viability Assay
2.5. Intracellular ROS Assay
2.6. Melanin Quantification
2.7. Western Blot
2.8. RNA Extraction and RT-PCR
2.9. Immunofluorescence Assay
2.10. siRNA Transfection
2.11. Statistical Analysis
3. Results
3.1. Ectoine Inhibited UVA-Induced ROS Generation in HaCaT Cells
3.2. Ectoine Suppressed POMC and α-MSH Expressions in UVA-Irradiated HaCaT Cells
3.3. Ectoine Downregulated Melanin and Tyrosinase Expression in α-MSH-Stimulated B16F10 Cells
3.4. Ectoine Facilitated Nrf2 Nuclear Translocation in HaCaT Cells
3.5. Ectoine Upregulated the Expression of HO-1, NQO-1, and γ-GCLC Proteins in HaCaT Cells
3.6. Various Signaling Pathways Were Involved in the Activation of Nrf2 in Ectoine Treated HaCaT Cells
3.7. Ectoine Mediated Anti-Melanogenic Effect was Suppressed due to the Knockdown of Nrf2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- del Giudice, P.; Yves, P. The widespread use of skin lightening creams in Senegal: A persistent public health problem in West Africa. Int. J. Dermatol. 2002, 41, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Dey, V.K. Misuse of topical corticosteroids: A clinical study of adverse effects. Indian Dermatol. Online J. 2014, 5, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final Report of the Safety Assessment of Kojic Acid as Used in Cosmetics. Int. J. Toxicol. 2010, 29, 244s–273s. [Google Scholar] [CrossRef]
- Mata, T.L.; Sanchez, J.P.; Oyanguren, J.D. Allergic contact dermatitis due to Kojic acid. Dermatitis 2005, 16, 89. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef]
- Lentzen, G.; Schwarz, T. Extremolytes: Natural compounds from extremophiles for versatile applications. Appl. Microbiol. Biotechnol. 2006, 72, 623–634. [Google Scholar] [CrossRef]
- Stepniewska, Z.; Goraj, W.; Kuzniar, A.; Pytlak, A.; Ciepielski, J.; Fraczek, P. Biosynthesis of Ectoine by the Methanotrophic Bacterial Consortium Isolated from Bogdanka Coalmine (Poland). Appl. Biochem. Microbiol. 2014, 50, 594–600. [Google Scholar] [CrossRef]
- Peters, P.; Galinski, E.A.; Truper, H.G. The Biosynthesis of Ectoine. Fems Microbiol. Lett. 1990, 71, 157–162. [Google Scholar] [CrossRef]
- Harishchandra, R.K.; Wulff, S.; Lentzen, G.; Neuhaus, T.; Galla, H.J. The effect of compatible solute ectoines on the structural organization of lipid monolayer and bilayer membranes. Biophys. Chem. 2010, 150, 37–46. [Google Scholar] [CrossRef]
- Meyer, S.; Schroter, M.A.; Hahn, M.B.; Solomun, T.; Sturm, H.; Kunte, H.J. Ectoine can enhance structural changes in DNA in vitro. Sci. Rep. 2017, 7, 7170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sydlik, U.; Peuschel, H.; Paunel-Gorgulu, A.; Keymel, S.; Kramer, U.; Weissenberg, A.; Kroker, M.; Seghrouchni, S.; Heiss, C.; Windolf, J.; et al. Recovery of neutrophil apoptosis by ectoine: A new strategy against lung inflammation. Eur. Respir. J. 2013, 41, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bünger, J.; Driller, H.J.; Martin, R. Use of Ectoine or Ectoine Derivatives in Cosmetic Formulations. U.S. Patent 6,602,514, 5 August 2003. [Google Scholar]
- Buenger, J.; Driller, H. Ectoin: An effective natural substance to prevent UVA-induced premature photoaging. Skin Pharmacol. Physiol. 2004, 17, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Reinelt, K.; Krutmann, J.; Bilstein, A. Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis: A randomised, comparator-controlled, intra-individual double-blind, multi-center trial. Skin Pharmacol. Physiol. 2014, 27, 57–65. [Google Scholar] [CrossRef]
- Kanapathipillai, M.; Lentzen, G.; Sierks, M.; Park, C.B. Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer’s beta-amyloid. FEBS Lett. 2005, 579, 4775–4780. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, A.; Benasher, E.; Eisenstein, M. Tetrahydropyrimidine Derivatives Inhibit Binding of a Tat-Like, Arginine-Containing Peptide, to Hiv Tar Rna in-Vitro. FEBS Lett. 1995, 367, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Bilstein, A.; Overhagen, S.; Géczi, L.; Baráth, Z.; Mösges, R. Effectiveness, Tolerability, and Safety of Ectoine-Containing Mouthwash Versus Those of a Calcium Phosphate Mouthwash for the Treatment of Chemotherapy-Induced Oral Mucositis: A Prospective, Active-Controlled, Non-interventional Study. Oncol. Ther. 2018, 6, 59–72. [Google Scholar]
- Unfried, K.; Krämer, U.; Sydlik, U.; Autengruber, A.; Bilstein, A.; Stolz, S.; Marini, A.; Schikowski, T.; Keymel, S.; Krutmann, J. Reduction of chronic lung inflammation by inhalation of the compatible solute ectoine: A population-based intervention study with elderly individuals. Pneumologie 2017, 71, S1–S125. [Google Scholar] [CrossRef]
- Yao, C.L.; Lin, Y.M.; Mohamed, M.S.; Chen, J.H. Inhibitory effect of ectoine on melanogenesis in B16-F0 and A2058 melanoma cell lines. Biochem. Eng. J. 2013, 78, 163–169. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Ho, Y.G.; Mathew, D.C.; Yen, H.R.; Chen, X.Z.; Yang, H.L. The in vitro and in vivo depigmenting activity of Coenzyme Q10 through the down-regulation of alpha-MSH signaling pathways and induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated skin keratinocytes. Biochem. Pharmacol. 2019, 164, 299–310. [Google Scholar] [CrossRef]
- Yang, H.L.; Lin, S.W.; Lee, C.C.; Lin, K.Y.; Liao, C.H.; Yang, T.Y.; Wang, H.M.; Huang, H.C.; Wu, C.R.; Hseu, Y.C. Induction of Nrf2-mediated genes by Antrodia salmonea inhibits ROS generation and inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Funct. 2015, 6, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Cawley, N.X.; Li, Z.; Loh, Y.P. 60 YEARS OF POMC: Biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J. Mol. Endocrinol. 2016, 56, T77–T97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGregor, D. Hydroquinone: An evaluation of the human risks from its carcinogenic and mutagenic properties. Crit. Rev. Toxicol. 2007, 37, 887–914. [Google Scholar] [CrossRef]
- Kolbe, L.; Kligman, A.M.; Schreiner, V.; Stoudemayer, T. Corticosteroid-induced atrophy and barrier impairment measured by non-invasive methods in human skin. Skin Res. Technol. 2002, 7, 73–77. [Google Scholar] [CrossRef]
- Desmedt, B.; Courselle, P.; De Beer, J.O.; Rogiers, V.; Grosber, M.; Deconinck, E.; De Paepe, K. Overview of skin whitening agents with an insight into the illegal cosmetic market in Europe. J. Eur. Acad. Dermatol. 2016, 30, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Graf, R.; Anzali, S.; Buenger, J.; Pfluecker, F.; Driller, H. The multifunctional role of ectoine as a natural cell protectant. Clin. Dermatol. 2008, 26, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Henri, P.; Beaumel, S.; Guezennec, A.; Poumes, C.; Stoebner, P.E.; Stasia, M.J.; Guesnet, J.; Martinez, J.; Meunier, L. MC1R expression in HaCaT keratinocytes inhibits UVA-induced ROS production via NADPH oxidase- and cAMP-dependent mechanisms. J. Cell Physiol. 2012, 227, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, K.; Kauser, S.; Pritchard, L.E.; Warhurst, A.; Oliver, R.L.; Slominski, A.; Wei, E.T.; Thody, A.J.; Tobin, D.J.; White, A. Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. FASEB J. 2007, 21, 1844–1856. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.Y.; Pang, J.H.; Huang, S.T. Inhibition of melanogenesis in murine B16/F10 melanoma cells by Ligusticum sinensis Oliv. Am. J. Chin. Med. 2006, 34, 523–533. [Google Scholar] [CrossRef]
- Gegotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Marrot, L.; Jones, C.; Perez, P.; Meunier, J.R. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008, 21, 79–88. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hseu, Y.-C.; Chen, X.-Z.; Vudhya Gowrisankar, Y.; Yen, H.-R.; Chuang, J.-Y.; Yang, H.-L. The Skin-Whitening Effects of Ectoine via the Suppression of α-MSH-Stimulated Melanogenesis and the Activation of Antioxidant Nrf2 Pathways in UVA-Irradiated Keratinocytes. Antioxidants 2020, 9, 63. https://doi.org/10.3390/antiox9010063
Hseu Y-C, Chen X-Z, Vudhya Gowrisankar Y, Yen H-R, Chuang J-Y, Yang H-L. The Skin-Whitening Effects of Ectoine via the Suppression of α-MSH-Stimulated Melanogenesis and the Activation of Antioxidant Nrf2 Pathways in UVA-Irradiated Keratinocytes. Antioxidants. 2020; 9(1):63. https://doi.org/10.3390/antiox9010063
Chicago/Turabian StyleHseu, You-Cheng, Xuan-Zao Chen, Yugandhar Vudhya Gowrisankar, Hung-Rong Yen, Jing-Yuan Chuang, and Hsin-Ling Yang. 2020. "The Skin-Whitening Effects of Ectoine via the Suppression of α-MSH-Stimulated Melanogenesis and the Activation of Antioxidant Nrf2 Pathways in UVA-Irradiated Keratinocytes" Antioxidants 9, no. 1: 63. https://doi.org/10.3390/antiox9010063