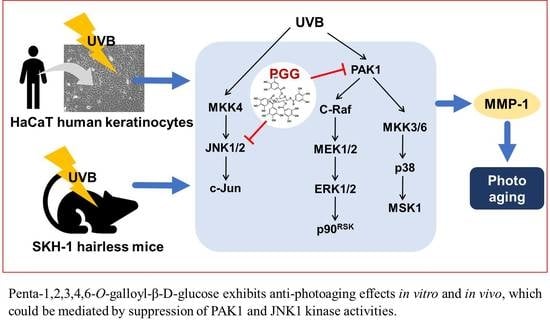
Penta-1,2,3,4,6-O-Galloyl-β-d-Glucose Inhibits UVB-Induced Photoaging by Targeting PAK1 and JNK1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Culture and UVB Irradiation
2.3. In Vitro MMP-1 Activity Assay
2.4. MTT Assay
2.5. Western Blot Analysis
2.6. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.7. In Vitro PAK1 and JNK1 Kinase Assays
2.8. In Vitro PAK1 and JNK1 Binding Assays
2.9. ATP and PGG Competition Assays
2.10. Experimental Animals
2.11. UV Irradiation of Hairless Mice
2.12. Preparation of Skin Lysates
2.13. Generation of Replicas and Image Analysis
2.14. Measurement of Transepidermal Water Loss (TEWL)
2.15. Histological Examination
2.16. Immunohistochemical Staining
2.17. Statistical Analysis
3. Results
3.1. PGG Inhibits UVB-Induced MMP-1 Expression in HaCaT Cells
3.2. PGG Suppresses UVB-Induced RAF-MEK-ERK-p90RSK, MKK3/6-p38-MSK1, and c-Jun, but not MKK4/7-JNK in HaCaT Cells
3.3. PAK1 is Involved in UVB-Induced MMP-1 Upregulation by Modulating the Activation of ERK and p38 Pathways
3.4. PGG Inhibits PAK1 and JNK1 Kinase Activities In Vitro
3.5. PGG Binds with PAK1 in an ATP-Competitive Manner and JNK1 in an ATP-Independent Manner
3.6. PGG Inhibits UVB-Induced Photoaging Symptoms In Vivo
3.7. PGG Suppresses UVB-Induced Epidermal Thickening In Vivo
3.8. PGG Increases UVB-Induced Type 1 Collagen Degradation and Inhibits UVB-Induced MMP-13 Expression In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H. Photoaging in Asians. Photodermatol. Photoimmunol. Photomed. 2003, 19, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Jung, S.K.; Byun, S.; Lee, E.J.; Hwang, J.A.; Seo, S.G.; Kim, Y.A.; Yu, J.G.; Lee, K.W.; Lee, H.J. Luteolin suppresses UVB-induced photoageing by targeting JNK1 and p90RSK2. J. Cell. Mol. Med. 2013, 17, 672–680. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Chung, J.H.; Hanft, V.N.; Kang, S. Aging and photoaging. J. Am. Acad. Dermatol. 2003, 49, 690–697. [Google Scholar] [CrossRef]
- Varani, J.; Hattori, Y.; Chi, Y.; Schmidt, T.; Perone, P.; Zeigler, M.E.; Fader, D.J.; Johnson, T.M. Collagenolytic and gelatinolytic matrix metalloproteinases and their inhibitors in basal cell carcinoma of skin: Comparison with normal skin. Br. J. Cancer 2000, 82, 657–665. [Google Scholar] [CrossRef]
- Gelse, K.; Poschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.E.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Chen, W.; Bowden, G.T. Activation of p38 MAP kinase and ERK are required for ultraviolet-B induced c-fos gene expression in human keratinocytes. Oncogene 1999, 18, 7469–7476. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Sells, M.A.; Knaus, U.G.; Bagrodia, S.; Ambrose, D.M.; Bokoch, G.M.; Chernoff, J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997, 7, 202–210. [Google Scholar] [CrossRef]
- Bokoch, G.M. Biology of the p21-activated kinases. Annu. Rev. Biochem. 2003, 72, 743–781. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Sun, H.Y.; Diaz, B.; Barnard, D.; Miao, W.Y.; Bagrodia, S.; Marshall, M.S. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 1998, 396, 180–183. [Google Scholar] [CrossRef]
- Slack-Davis, J.K.; Eblen, S.T.; Zecevic, M.; Boerner, S.A.; Tarcsafalvi, A.; Diaz, H.B.; Marshall, M.S.; Weber, M.J.; Parsons, J.T.; Catling, A.D. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 2003, 162, 281–291. [Google Scholar] [CrossRef]
- Ong, C.C.; Jubb, A.M.; Jakubiak, D.; Zhou, W.; Rudolph, J.; Haverty, P.M.; Kowanetz, M.; Yan, Y.; Tremayne, J.; Lisle, R.; et al. P21-activated kinase 1 (PAK1) as a therapeutic target in BRAF wild-type melanoma. J. Natl. Cancer Inst. 2013, 105, 606–607. [Google Scholar] [CrossRef]
- Jeon, W.K.; Lee, J.H.; Kim, H.K.; Lee, A.Y.; Lee, S.O.; Kim, Y.S.; Ryu, S.Y.; Kim, S.Y.; Lee, Y.J.; Ko, B.S. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 2006, 106, 62–69. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.J.; Kim, D.W.; Kim, G.Y.; Kim, J.K.; Gebru, Y.A.; Choi, H.S.; Kim, Y.H.; Kim, M.K. Changes of Phytochemical Components (Urushiols, Polyphenols, Gallotannins) and Antioxidant Capacity during Fomitella fraxinea(-)Mediated Fermentation of Toxicodendron vernicifluum Bark. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Lee, H.H.; Ho, C.T.; Lin, J.K. Theaflavin-3,3′-digallate and penta-O-galloyl-beta-D-glucose inhibit rat liver microsomal 5 alpha-reductase activity and the expression of androgen receptor in LNCaP prostate cancer cells. Carcinogenesis 2004, 25, 1109–1118. [Google Scholar] [CrossRef]
- Hula, K.T.; Way, T.D.; Lin, J.K. Pentagalloylglucose inhibits estrogen receptor alpha by lysosome-dependent depletion and modulates ErbB/PI3K/Akt pathway in human breast cancer MCF-7 cells. Mol. Carcinog. 2006, 45, 551–560. [Google Scholar] [CrossRef]
- Hu, H.; Lee, H.J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.O.; Kim, S.H.; Lu, J. Penta-1,2,3,4,6-O-galloyl-beta-D-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008, 7, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, N.J.; Jeong, S.J.; Park, Y.; Jung, D.B.; Koh, W.; Lee, E.O.; Ahn, K.S.; Lu, J.; Kim, S.H. Oral administration of penta-O-galloyl-beta-D-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis 2011, 32, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Sancheti, S.A.; Sancheti, S.S.; Um, B.H.; Yu, S.M.; Seo, S.Y. Effect of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose on elastase and hyaluronidase activities and its type II collagen expression. Acta Pol. Pharm. 2010, 67, 145–150. [Google Scholar]
- Kim, B.H.; Choi, M.S.; Lee, H.G.; Lee, S.H.; Noh, K.H.; Kwon, S.; Jeong, A.J.; Lee, H.; Yi, E.H.; Park, J.Y.; et al. Photoprotective Potential of Penta-O-Galloyl-beta-DGlucose by Targeting NF-kappaB and MAPK Signaling in UVB Radiation-Induced Human Dermal Fibroblasts and Mouse Skin. Mol. Cells 2015, 38, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.G.; Kim, Y.A.; Kim, J.E.; Baek, S.; Lee, S.Y.; Lee, C.C.; Chen, H.; Kim, J.R.; Kwon, J.Y.; Bode, A.M.; et al. PKCiota is a target of 7,8,4′-trihydroxyisoflavone for the suppression of UVB-induced MMP-1 expression. Exp. Dermatol. 2017. [Google Scholar] [CrossRef]
- Shin, J.; Kim, J.E.; Pak, K.J.; Kang, J.I.; Kim, T.S.; Lee, S.Y.; Yeo, I.H.; Park, J.H.; Kim, J.H.; Kang, N.J.; et al. A Combination of Soybean and Haematococcus Extract Alleviates Ultraviolet B-Induced Photoaging. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.S.; Kang, N.J.; Bode, A.M.; Dong, Z.G.; et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef]
- Moon, H.I.; Chung, J.H. Meso-dihydroguaiaretic acid from Machilus thunbergii SIEB et ZUCC., and its effects on the expression of matrix metalloproteinase-2, 9 cause by ultraviolet irradiated cultured human keratinocyte cells (HaCaT). Biol. Pharm. Bull. 2005, 28, 2176–2179. [Google Scholar] [CrossRef]
- Jean, C.; Bogdanowicz, P.; Haure, M.J.; Castex-Rizzi, N.; Fournie, J.J.; Laurent, G. UVA-activated synthesis of metalloproteinases 1, 3 and 9 is prevented by a broad-spectrum sunscreen. Photodermatol. Photoimmunol. Photomed. 2011, 27, 318–324. [Google Scholar] [CrossRef]
- Wang, X.Y.; Bi, Z.G. UVB-irradiated human keratinocytes and interleukin-1alpha indirectly increase MAP kinase/AP-1 activation and MMP-1 production in UVA-irradiated dermal fibroblasts. Chin. Med. J. 2006, 119, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Rider, L.; Oladimeji, P.; Diakonova, M. PAK1 regulates breast cancer cell invasion through secretion of matrix metalloproteinases in response to prolactin and three-dimensional collagen IV. Mol. Endocrinol. 2013, 27, 1048–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, L.L.; Lam, I.P.; Wong, T.Y.; Lai, W.L.; Liu, H.F.; Yeung, L.L.; Ching, Y.P. IPA-3 inhibits the growth of liver cancer cells by suppressing PAK1 and NF-kappaB activation. PLoS ONE 2013, 8, e68843. [Google Scholar] [CrossRef] [Green Version]
- Bissett, D.L.; Hannon, D.P.; Orr, T.V. An animal model of solar-aged skin: Histological, physical, and visible changes in UV-irradiated hairless mouse skin. Photochem. Photobiol. 1987, 46, 367–378. [Google Scholar] [CrossRef]
- Song, J.H.; Bae, E.Y.; Choi, G.; Hyun, J.W.; Lee, M.Y.; Lee, H.W.; Chae, S. Protective effect of mango (Mangifera indica L.) against UVB-induced skin aging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2013, 29, 84–89. [Google Scholar] [CrossRef]
- Pyun, H.B.; Kim, M.; Park, J.; Sakai, Y.; Numata, N.; Shin, J.Y.; Shin, H.J.; Kim, D.U.; Hwang, J.K. Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev. Nutr. Food Sci. 2012, 17, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Gilchrest, B.A. A review of skin ageing and its medical therapy. Br. J. Dermatol. 1996, 135, 867–875. [Google Scholar] [CrossRef]
- Wen, K.C.; Fan, P.C.; Tsai, S.Y.; Shih, I.C.; Chiang, H.M. Ixora parviflora Protects against UVB-Induced Photoaging by Inhibiting the Expression of MMPs, MAP Kinases, and COX-2 and by Promoting Type I Procollagen Synthesis. Evid. Based Complement. Altern. Med. 2012, 2012, 417346. [Google Scholar] [CrossRef]
- Wu, N.; Jansen, E.D.; Davidson, J.M. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J. Investig. Dermatol. 2003, 121, 926–932. [Google Scholar] [CrossRef] [Green Version]
- Madlener, M.; Parks, W.C.; Werner, S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp. Cell Res. 1998, 242, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Freije, J.M.; Diez-Itza, I.; Balbin, M.; Sanchez, L.M.; Blasco, R.; Tolivia, J.; Lopez-Otin, C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J. Biol. Chem. 1994, 269, 16766–16773. [Google Scholar] [PubMed]
- Park, J.E.; Pyun, H.B.; Woo, S.W.; Jeong, J.H.; Hwang, J.K. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol. Photoimmunol. Photomed. 2014, 30, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011, 61, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Gum, R.; Wang, H.; Lengyel, E.; Juarez, J.; Boyd, D. Regulation of 92 kDa type IV collagenase expression by the jun aminoterminal kinase- and the extracellular signal-regulated kinase-dependent signaling cascades. Oncogene 1997, 14, 1481–1493. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Kumar, R.; Gururaj, A.E.; Barnes, C.J. p21-activated kinases in cancer. Nat. Rev. Cancer 2006, 6, 459–471. [Google Scholar] [CrossRef]
- Jin, X.J.; Kim, E.J.; Oh, I.K.; Kim, Y.K.; Park, C.H.; Chung, J.H. Prevention of UV-induced skin damages by 11,14,17-eicosatrienoic acid in hairless mice in vivo. J. Korean Med. Sci. 2010, 25, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Lee, M.J.; Lee, S.R.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice. Mech. Ageing Dev. 2005, 126, 1170–1177. [Google Scholar] [CrossRef]
- Kim, H.H.; Cho, S.; Lee, S.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J. Lipid Res. 2006, 47, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Kim, J.H.; Nam, K.T.; Lee, S.H. Molecular events associated with apoptosis and proliferation induced by ultraviolet-B radiation in the skin of hairless mice. J. Dermatol. Sci. 2003, 32, 171–179. [Google Scholar] [CrossRef]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008, 7, s12–s16. [Google Scholar] [PubMed]
- Dumas, E.O.; Pollack, G.M. Opioid tolerance development: A pharmacokinetic/pharmacodynamic perspective. AAPS J. 2008, 10, 537–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bespalov, A.; Muller, R.; Relo, A.L.; Hudzik, T. Drug Tolerance: A Known Unknown in Translational Neuroscience. Trends Pharmacol. Sci. 2016, 37, 364–378. [Google Scholar] [CrossRef] [PubMed]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-A.; Lee, J.-E.; Kim, J.H.; Lee, H.-J.; Kang, N.J. Penta-1,2,3,4,6-O-Galloyl-β-d-Glucose Inhibits UVB-Induced Photoaging by Targeting PAK1 and JNK1. Antioxidants 2019, 8, 561. https://doi.org/10.3390/antiox8110561
Kim J-A, Lee J-E, Kim JH, Lee H-J, Kang NJ. Penta-1,2,3,4,6-O-Galloyl-β-d-Glucose Inhibits UVB-Induced Photoaging by Targeting PAK1 and JNK1. Antioxidants. 2019; 8(11):561. https://doi.org/10.3390/antiox8110561
Chicago/Turabian StyleKim, Ji-An, Jae-Eun Lee, Ji Hye Kim, Hyo-Jeong Lee, and Nam Joo Kang. 2019. "Penta-1,2,3,4,6-O-Galloyl-β-d-Glucose Inhibits UVB-Induced Photoaging by Targeting PAK1 and JNK1" Antioxidants 8, no. 11: 561. https://doi.org/10.3390/antiox8110561