Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis
Abstract
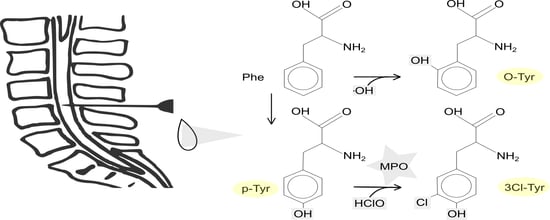
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Standards and Reagents
2.3. Sample Preparation and Analysis Employing Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
2.4. Statistical Analysis
2.5. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Child Mortality Collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1725–1774. [Google Scholar] [CrossRef]
- Edmond, K.; Clark, A.; Korczak, V.S.; Sanderson, C.; Griffiths, U.K.; Rudan, I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2010, 10, 317–328. [Google Scholar] [CrossRef]
- Gerber, J.; Nau, R. Mechanisms of injury in bacterial meningitis. Curr. Opin. Neurol. 2010, 23, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liechti, F.D.; Grandgirard, D.; Leib, S.L. Bacterial meningitis: Insights into pathogenesis and evaluation of new treatment options: A perspective from experimental studies. Future Microbiol. 2015, 10, 1195–1213. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Simoes, L.R.; Elias, S.G.; Quevedo, J. Role of oxidative stress in the pathophysiology of pneumococcal meningitis. Oxid. Med. Cell. Longev. 2013, 2013, 371465. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Collodel, A.; Moreira, A.P.; Almeida, S.M. Pathophysiology of acute meningitis caused by Streptococcus pneumoniae and adjunctive therapy approaches. Arq. Neuropsiquiatr. 2012, 70, 366–372. [Google Scholar] [CrossRef]
- Radi, R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y. Neutrophil myeloperoxidase and its substrates: Formation of specific markers and reactive compounds during inflammation. J. Clin. Biochem. Nutr. 2016, 58, 99–104. [Google Scholar] [CrossRef]
- Srivastava, R.; Lohokare, R.; Prasad, R. Oxidative stress in children with bacterial meningitis. J. Trop. Pediatr. 2013, 59, 305–308. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, J.; Torres-Cuevas, I.; Quintas, G.; Rook, D.; van Goudoever, J.B.; Cubells, E.; Asensi, M.; Lliso, I.; Nunez, A.; Vento, M.; et al. Assessment of oxidative damage to proteins and DNA in urine of newborn infants by a validated UPLC-MS/MS approach. PLoS ONE 2014, 9, e93703. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Fu, S.; Wang, H.; Dean, R.T. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic. Biol. Med. 1999, 27, 1151–1163. [Google Scholar] [CrossRef]
- Klein, M.; Koedel, U.; Pfister, H.W. Oxidative stress in pneumococcal meningitis: A future target for adjunctive therapy? Prog. Neurobiol. 2006, 80, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, T.; Roine, I.; Cruzeiro, M.L.; Pitkaranta, A.; Kataja, M.; Peltola, H. Slow initial beta-lactam infusion and oral paracetamol to treat childhood bacterial meningitis: A randomised, controlled trial. Lancet Infect. Dis. 2011, 11, 613–621. [Google Scholar] [CrossRef]
- Chafer-Pericas, C.; Stefanovic, V.; Sanchez-Illana, A.; Escobar, J.; Cernada, M.; Cubells, E.; Nunez-Ramiro, A.; Andersson, S.; Vento, M.; Kuligowski, J. Novel biomarkers in amniotic fluid for early assessment of intraamniotic infection. Free Radic. Biol. Med. 2015, 89, 734–740. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Simoes, L.R.; Dagostin, V.S.; Generoso, J.S.; Rezin, G.T.; Florentino, D.; Muniz, J.P.; Collodel, A.; Petronilho, F.; Quevedo, J.; et al. Temporal changes of oxidative stress markers in Escherichia coli K1-induced experimental meningitis in a neonatal rat model. Neurosci. Lett. 2017, 653, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Haruta, T.; Todoroki, Y.; Hiraoka, M.; Noiri, E.; Maeda, M.; Mayumi, M. Oxidant and antioxidant activities in childhood meningitis. Life Sci. 2002, 71, 2797–2806. [Google Scholar] [CrossRef]
- Ray, G.; Aneja, S.; Jain, M.; Batra, S. Evaluation of free radical status in CSF in childhood meningitis. Ann. Trop. Paediatr. 2000, 20, 115–120. [Google Scholar] [CrossRef]
- Molnar, G.A.; Kun, S.; Selley, E.; Kertész, M.; Szélig, L.; Csontos, C.; Böddi, K.; Bogár, L.; Miseta, A.; Wittmann, I. Role of tyrosine isomers in acute and chronic diseases leading to oxidative stress—A review. Curr. Med. Chem. 2016, 23, 667–685. [Google Scholar] [CrossRef]
- Strasser, B.; Sperner-Unterweger, B.; Fuchs, D.; Gostner, J.M. Mechanisms of inflammation-associated depression: Immune influences on tryptophan and phenylalanine metabolisms. Curr. Top. Behav. Neurosci. 2017, 31, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Mook-Kanamori, B.B.; Geldhoff, M.; van der Poll, T.; van de Beek, D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 2011, 24, 557–591. [Google Scholar] [CrossRef] [PubMed]
- Roine, I.; Peltola, H.; Fernandez, J.; Zavala, I.; Gonzalez Mata, A.; Gonzalez Ayala, S.; Arbo, A.; Bologna, R.; Mino, G.; Goyo, J.; et al. Influence of admission findings on death and neurological outcome from childhood bacterial meningitis. Clin. Infect. Dis. 2008, 46, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Miric, D.; Katanic, R.; Kisic, B.; Zoric, L.; Miric, B.; Mitic, R.; Dragojevic, I. Oxidative stress and myeloperoxidase activity during bacterial meningitis: Effects of febrile episodes and the BBB permeability. Clin. Biochem. 2010, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Lemos, J.C.; Generoso, J.S.; Cipriano, A.L.; Milioli, G.L.; Marcelino, D.M.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Vilela, M.C.; et al. Oxidative stress, cytokine/chemokine and disruption of blood-brain barrier in neonate rats after meningitis by Streptococcus agalactiae. Neurochem. Res. 2011, 36, 1922–1930. [Google Scholar] [CrossRef]
- Kastenbauer, S.; Koedel, U.; Becker, B.F.; Pfister, H.W. Oxidative stress in bacterial meningitis in humans. Neurology 2002, 58, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Haruta, T.; Ono, N.; Kobata, R.; Fukumoto, Y.; Hiraoka, M.; Mayumi, M. Oxidative stress in childhood meningitis: Measurement of 8-hydroxy-2′-deoxyguanosine concentration in cerebrospinal fluid. Redox Rep. 2000, 5, 295–298. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Kuligowski, J.; Carcel, M.; Cháfer-Pericás, C.; Asensi, M.; Solberg, R.; Cubells, E.; Nuñez, A.; Saugstad, O.D.; Vento, M.; et al. Protein-bound tyrosine oxidation, nitration and chlorination by-products assessed by ultraperformance liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2016, 913, 104–110. [Google Scholar] [CrossRef]
- Ipson, B.R.; Fisher, A.L. Roles of the tyrosine isomers meta-tyrosine and ortho-tyrosine in oxidative stress. Ageing Res. Rev. 2016, 27, 93–107. [Google Scholar] [CrossRef]


| Analyte | Retention Time ± s [min] | Calibration Range | R2 | y = a + bx | m/z Parent Ion | Cone [V] | Daughter Ion | Internal Standard | ||
|---|---|---|---|---|---|---|---|---|---|---|
| a [nm–1] | b [nM] | CE [eV] | m/z Quantification | |||||||
| Phenylalanine (Phe) | 2.32 ± 0.01 | 0.2–400 µM | 0.954 | 16.4 | 0.01 | 166.1 | 20 | 20 | 91.0 | Phe-D5 |
| Para-tyrosine (p-Tyr) | 1.01 ± 0.01 | 0.2–400 µM | 0.999 | 38.8 | 0.03 | 182.1 | 20 | 10 | 91.0 | p-Tyr-d2 |
| Ortho-tyrosine (o-Tyr) | 1.80 ± 0.01 | 1–2000 nM | 0.999 | –0.1 | 0.15 | p-Tyr-d2 | ||||
| 3-chlorotyrosine (3Cl-Tyr) | 1.90 ± 0.01 | 2–4000 nM | 0.999 | –2.1 | 1.68 | 216.0 | 20 | 15 | 170.0 | Phe-D5 |
| 3-nitrotyrosine (3NO2-Tyr) | 2.33 ± 0.01 | 1–2000 nM | 0.995 | 8.0 | 2.87 | 227.1 | 25 | 10 | 181.0 | Phe-D5 |
| 2-deoxyguanosine (2dG) | 1.45 ± 0.03 | 1–2000 nM | 0.999 | –0.3 | 0.41 | 268.0 | 25 | 15 | 152.0 | 2dG-13C15N |
| 8-oxo-2′-deoxyguanosine (8OHdG) | 2.04 ± 0.02 | 1–500 nM | 0.999 | 0.1 | 0.35 | 284.0 | 30 | 15 | 168.0 | 8OHdG-13C15N |
| Phenylalanine-d5 (Phe-D5) | 2.32 ± 0.01 | - | - | - | 171.5 | 30 | 20 | 125.0 | - | |
| p-Tyrosine-d2 (p-Tyr-D2) | 1.01 ± 0.02 | - | - | - | 184.1 | 20 | 10 | 138.1 | - | |
| 2-Deoxyguanosine-13C15N (2dG-13C15N) | 1.45 ± 0.02 | - | - | - | 271.0 | 15 | 10 | 155.0 | - | |
| 8-Oxo-2′-deoxyguanosine-13C15N (8OHdG-13C15N) | 2.04 ± 0.03 | - | - | - | 287.0 | 30 | 15 | 171.0 | - | |
| VARIABLE | BM (n = 79) |
|---|---|
| Age in months, median (IQR) | 12 (7–42) |
| Weight for age below—2SD | 19 (24%) |
| Duration of illness days, median (IQR) | 4 (3–7) |
| Previous antibiotics * | 30/74 (41%) |
| Glasgow coma score, median (IQR) | 11 (7–14) ᵃ |
| Another focus of infection | 19 (24%) |
| Cerebrospinal fluid | |
| Leukocyte count (×10⁶/L), median (IQR) | 1740 (353–3515) |
| Glucose concentration (mg/dL), median (IQR) | 17 (9–26) ᵇ |
| Blood | |
| CRP # on day 1 or 2 (mg/L), median ** (IQR) | 154 (81–161) ᶜ |
| Glucose (mg/dL), median (IQR) *** | 85 (62–111) ᵈ |
| Hemoglobin day 1 or 2 (g/dL), median (IQR) | 7.5 (6–9) e |
| Causative agent | |
| Streptococcus pneumoniae | 40/79 (51%) |
| Haemophilus influenzae type b | 24/79 (30%) |
| Neisseria meningitidis | 11/79 (14%) |
| Other bacteria | 4/79 (5%) |
| VARIABLE | BM, Luanda (n = 79) | Control, Helsinki (n = 10) | p Value | Ratio BM/Contol |
|---|---|---|---|---|
| Phenylalanine (Phe) | 88,346 (51,535−166,316) | 6558.0 (5249.76−8473) | < 0.0001 | 13.5 |
| Para-tyrosine (p-Tyr) | 64,214 (31,197−152,125) | 13,239 (10,096−17,677) | < 0.0001 | 4.9 |
| Ortho-tyrosine (o-Tyr) | 162.12 (65.23−2194.9) | 0.02 (0.020) | < 0.0001 | 8100 |
| 3-chlorotyrosine (3Cl-Tyr) | 423.34 (134.84−1311.95) | 4.155 (4.155) | < 0.0001 | 102 |
| 3-nitrotyrosine (3NO₂-Tyr) | 90.48 (59.37−135.47) | 2.745 (1.181−4.61) | < 0.0001 | 32.7 |
| 2′deoxiguanosine (2dG) | 303.57 (91.32−1329.69) | 0.768 (0.768−3.538) | < 0.0001 | 395 |
| 8-oxo-2′-deoxyguanosine (8OHdG) | 3.895 (3.895−17.778) | 0.043 (0.043) | < 0.0001 | 90.6 |
| Ratio 3Cl-Tyr/p-Tyr | 0.007 (0.003−0.022) | 3.531 × 10−4 (2.498 × 10−4−0.001) | < 0.0001 | 19.8 |
| Ratio o-Tyr/Phe | 0.002 (0.001−0.013) | 3.4995 × 10−6 (2.85 × 10−6−6.665 × 10−5) | < 0.0001 | 572 |
| Ratio 3NO₂-Tyr/p-Tyr | 0.001 (0.001−0.002) | 2.235 × 10−4(1088 × 10−4–4.444 × 10−4) | < 0.0001 | 4.5 |
| Ratio 8OHdG/2dG | 0.025 (0.005−0.063) | 0.056 (0.012−0.056) | 0.428 | 0.45 |
| Streptococcus pneumoniae | Haemophilus influenzae | Neisseria meningitidis | p Value | |
|---|---|---|---|---|
| VARIABLE | n = 40 | n = 24 | n = 11 | |
| Phenylalanine (Phe) | 84,788 (43,702−131,709) | 90,950 (58,433−172,710) | 166,203 (46,372−219,475) | 0.4454 |
| Para-tyrosine (p-Tyr) | 59,871 (39,143−135,736) | 53,641 (28,876−112,299) | 153,636 (30,053−226,175) | 0.4902 |
| Ortho-tyrosine (o-Tyr) | 155.77 (60.51−1973.3) | 109.175 (50.75−1212.4) | 687.97 (95.335−6987.8) | 0.3539 |
| 3-chlorotyrosine (3Cl-Tyr) | 719.72 (289.6−2654.7) | 317.17 (104.79−1251.7) | 246.17 (101.36−497.2) | 0.0568 |
| 3-nitrotyrosine (3NO₂-Tyr) | 77.26 (52.49−125.0) | 91.91 (79.130−158.63) | 107.28 (57.94−144.99) | 0.39 |
| 2′-deoxiguanosine (2dG) | 521.94 (104.37−6199.1) | 161.98 (93.29−536.1) | 84 (54.26−281.38) | 0.0213 |
| 8-oxo-2′-deoxyguanosine (8OHdG) | 4.103 (3.895−23.83) | 3.895 (3.895−8.655) | 3.895 (3.895−8.619) | 0.3452 |
| Ratio o-Tyr/Phe | 0.001 (0.001−0.011) | 0.001 (0.001−0.013) | 0.011 (0.002−0.033) | 0.2968 |
| Ratio 3Cl-Tyr/p-Tyr | 0.012 (0.005−0.028) | 0.004 (0.002−0.018) | 0.002 (0.002−0.033) | 0.0021 |
| Ratio 3NO₂-Tyr/p-Tyr | 0.001 (0.001−0.002) | 0.002 (0.001−0.003) | 0.001 (0.001−0.002) | 0.287 |
| Ratio 8OHdG/2dG | 0.02 (0.004−0.059) | 0.025 (0.011−0.058) | 0.046 (0.026−0.072) | 0.1647 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugemalira, E.; Roine, I.; Kuligowski, J.; Sánchez-Illana, Á.; Piñeiro-Ramos, J.D.; Andersson, S.; Peltola, H.; Leite Cruzeiro, M.; Pelkonen, T.; Vento, M. Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis. Antioxidants 2019, 8, 441. https://doi.org/10.3390/antiox8100441
Rugemalira E, Roine I, Kuligowski J, Sánchez-Illana Á, Piñeiro-Ramos JD, Andersson S, Peltola H, Leite Cruzeiro M, Pelkonen T, Vento M. Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis. Antioxidants. 2019; 8(10):441. https://doi.org/10.3390/antiox8100441
Chicago/Turabian StyleRugemalira, Emilie, Irmeli Roine, Julia Kuligowski, Ángel Sánchez-Illana, José David Piñeiro-Ramos, Sture Andersson, Heikki Peltola, Manuel Leite Cruzeiro, Tuula Pelkonen, and Máximo Vento. 2019. "Protein Oxidation Biomarkers and Myeloperoxidase Activation in Cerebrospinal Fluid in Childhood Bacterial Meningitis" Antioxidants 8, no. 10: 441. https://doi.org/10.3390/antiox8100441