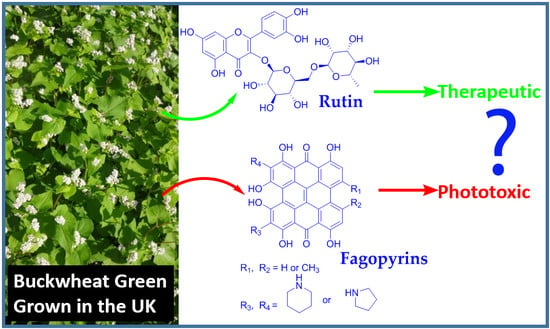
Antioxidant and Rutin Content Analysis of Leaves of the Common Buckwheat (Fagopyrum esculentum Moench) Grown in the United Kingdom: A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Phytochemical Analysis Methods and Chemicals
2.2. Growing Condition
2.3. Preparation of the Plant Material
2.4. HPLC Analysis
2.5. DPPH Radical Scavenging
2.6. Measurement of Reducing Power
2.7. Determination of Fagopyrins Level
2.8. Isolation of Rutin
3. Results and Discussion
3.1. The Rationale of Growing Buckwheat in European Countries Like the UK
3.2. Antioxidant and Reducing Power of Buckwheat Leaves Extract
3.3. Quantitative Determination of Rutin in Buckwheat Leaves
3.4. Isolation and Characterization of Rutin from Buckwheat Leaves
3.5. Fagopyrins Content Analysis of Buckwheat Leaves
3.6. General Summary and Conclusion
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and biofunctional properties of buckwheat: a review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Li, S.Q.; Zhang, Q.H. Advances in the development of functional foods from buckwheat. Crit. Rev. Food Sci. Nutr. 2001, 41, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Guo, Y.Z.; Chen, Q.F.; Yang, L.Y.; Huang, Y.H. Analyses of the seed protein contents on the cultivated and wild buckwheat Fagopyrum esculentum resources. Genet. Resour. Crop Evol. 2007, 54, 1465–1472. [Google Scholar] [CrossRef]
- Eggum, B.O.; Kreft, I.; Javornik, B. Chemical composition and protein quality of buckwheat (Fagopyrum esculentum Moench). Plant Foods Hum. Nutr. 1980, 30, 175–179. [Google Scholar] [CrossRef]
- Tahir, I.; Farooq, S. Grain composition in some buckwheat cultivars (Fagopyrum spp.) with particular reference to protein fractions. Plant Foods Hum. Nutr. 1985, 35, 153–158. [Google Scholar] [CrossRef]
- Skrabanja, V.; Liljeberg Elmståhl, H.G.; Kreft, I.; Björck, I.M. Nutritional properties of starch in buckwheat products: studies in vitro and in vivo. J. Agric. Food Chem. 2001, 49, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, M.N.; Giuliotti, L.; Pasqua, C.; Gatta, D.; Bagliacca, M. Buckwheat bran (Fagopyrum esculentum) as partial replacement of corn and soybean meal in the laying hen diet. Ital. J. Anim. Sci. 2012, 11, 9–12. [Google Scholar] [CrossRef]
- Prakash, S.; Yadav, K. Buckwheat (Fagopyrum esculentum) as a functional food: A Nutraceutical Pseudocereal. Int. J. Curr. Trend. Pharmacobiol. Med. Sci. 2016, 1, 1–15. [Google Scholar]
- Paulickova, L.; Vyb’alova, K.; Holasova, M.; Fiedlerova, V.; Vavreinova, S. Buckwheat as functional food. In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2004; pp. 587–592. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.579.6561&rep=rep1&type=pdf (accessed on 10 May 2019).
- Dražić, S.; Glamočlija, Đ.; Ristić, M.; Dolijanović, Ž.; Dražić, M.; Pavlović, S.; Jaramaz, M.; Jaramaz, D. Effect of environment of the rutin content in leaves of Fagopyrum esculentum Moench. Plant Soil Environ. 2016, 62, 261–265. [Google Scholar] [CrossRef]
- Kreft, S.; Jane, D. The content of fagopyrin and polyphenols in common and tartary buckwheat sprouts. Acta Pharm. 2013, 63, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, F.R. The occurrence and industrial production of rutin in Southeastern Australia. Econ. Bot. 1964, 18, 195–253. [Google Scholar] [CrossRef]
- FAOSTAT (Statistics Division of Food and Agriculture Organization of the United Nations). Buckwheat. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 May 2019).
- Habtemariam, S.; Jackson, C. Antioxidant and cytoprotective activity of leaves of Peltiphyllum peltatum (Torr.). Engl. Food Chem. 2007, 105, 498–503. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant activity of knipholone anthrone. Food Chem. 2007, 102, 1042–1047. [Google Scholar] [CrossRef]
- Habtemariam, S.; Varghese, G.K. Extractability of Rutin in Herbal Tea Preparations of Moringa stenopetala Leaves. Beverages 2015, 19, 169–182. [Google Scholar] [CrossRef]
- Habtemariam, S. Investigation into the antioxidant and antidiabetic potential of Moringa stenopetala: Identification of the active principles. Nat. Prod. Commun. 2015, 10, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Gabr, A.M.; Smetanska, I.; Kosyan, A. Pigments, phenolic contents and antioxidant activity of buckwheat seedlings under in vivo and in vitro conditions. In Climate Change: Challenges and Opportunities in Agriculture, Proceedings of the AGRISAFE Final Conference, Budapest, Hungary, 21–23 March 2011; Agricultural Research Institute of the Hungarian Academy of Sciences: Budapest, Hungary, 2011; pp. 348–352. [Google Scholar]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Zielinski, H.; Michalska, A.; Amigo-Benavent, M.; del Castillo, M.D.; Piskula, M.K. Changes in protein quality and antioxidant properties of buckwheat seeds and groats induced by roasting. J. Agric. Food Chem. 2009, 57, 4771–4776. [Google Scholar] [CrossRef]
- Kalinova, J.; Triska, J.; Vrchotova, N. Distribution of Vitamin E, squalene, epicatechin, and rutin in common buckwheat plants (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2006, 54, 5330–5335. [Google Scholar] [CrossRef]
- Ohara, T.; Ohinata, H.; Muramatsu, N.; Matsuhashi, T. Determination of rutin in buckwheat foods by high performance liquid chromatography. Nippon. Shokuhin Kogyo Gakkaishi 1989, 36, 114120. [Google Scholar] [CrossRef]
- Habtemariam, S.; Varghese, G.K. Antioxidant, anti-alpha-glucosidase and pancreatic beta-cell protective effects of methanolic extract of Ensete superbum Cheesm seeds. Asian Pac. J. Trop. Biomed. 2017, 7, 121–125. [Google Scholar] [CrossRef]
- Habtemariam, S.; Cowley, R.A. Antioxidant and anti-α-glucosidase compounds from the rhizome of Peltiphyllum peltatum (Torr.). Engl. Phytother. Res. 2012, 26, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Lentini, G.; Habtemariam, S. Phytochemical, antioxidant and anti-alpha-glucosidase activity evaluations of Bergenia cordifolia. Phytother. Res. 2012, 26, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Activity-guided isolation and identification of free radical-scavenging components from ethanolic extract of boneset (eaves of Eupatorium perfoliatum). Nat. Prod. Commun. 2008, 3, 1317–1320. [Google Scholar]
- Habtemariam, S. The African and Arabian Moringa Species: Chemistry, Bioactivity and Therapeutic Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–88. [Google Scholar]
- Benković, E.T.; Kreft, S. Fagopyrins and Protofagopyrins: Detection, analysis, and potential phototoxicity in buckwheat. J. Agric. Food Chem. 2015, 63, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Stojilkovski, K.; Kočevar Glavač, N.; Kreft, S.; Kreft, I. Fagopyrin and flavonoids content in common, tartary, and cymosum buckwheat. J. Food Comp. Anal. 2013, 32, 126–130. [Google Scholar] [CrossRef]
- Theurer, C.; Gruetzner, K.I.; Freeman, S.J.; Koetter, U. In vitro phototoxicity of hypericin, fagopyrin rich, and fagopyrin free buckwheat herb extracts. Pharm. Pharmacol. Lett. 1997, 7, 113–115. [Google Scholar]
- Eguchi, K.; Anase, T.; Osuga, H. Development of a high-performance liquid chromatography method to determine the fagopyrin content of tartary buckwheat (Fagopyrum tataricum Gaertn.) and common buckwheat (F. esculentum Moench). Plant Prod. Sci. 2009, 12, 475–480. [Google Scholar]
- Hinneburg, I.; Neubert, R.H.H. Influence of extraction parameters on the phytochemical characteristics of extracts from buckwheat (Fagopyrum esculentum) herb. J. Agric. Food Chem. 2005, 53, 3–7. [Google Scholar] [CrossRef]
- Glavač, N.K.; Stojilkovski, K.; Kreft, S.; Park, C.H.; Kreft, I. Determination of fagopyrins, rutin, and quercetin in Tartary buckwheat products. LWT Food Sci. Technol. 2017, 79, 423–427. [Google Scholar] [CrossRef]
- Habtemariam, S.; Lentini, G. The therapeutic potential of rutin for diabetes: An update. Mini Rev. Med. Chem. 2015, 15, 524–528. [Google Scholar] [CrossRef]
- Habtemariam, S. Rutin as a natural therapy for Alzheimer’s disease: Insights into its mechanisms of action. Curr. Med. Chem. 2016, 23, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Belai, A. Natural Therapies of the inflammatory bowel disease: The case of rutin and its aglycone, quercetin. Mini Rev. Med. Chem. 2018, 18, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The African Moringa is to change the lives of millions in Ethiopia and far beyond. Asian Pac. J. Trop. Biomed. 2016, 6, 355–356. [Google Scholar] [CrossRef]
- Kalinova, J.; Dadakova, E. Varietal differences of rutin in common buckwheat (Fagopyrum esculentum Moench) determined by micellar electrokinetic capillary chromatography. In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2004; pp. 719–722. [Google Scholar]
- Fabjan, N.; Rode, N.; Košir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [PubMed]
- Suzuki, T.; Honda, Y.; Mukasa, Y. Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 2005, 168, 1303–1307. [Google Scholar] [CrossRef]
- Ohsawa, R.; Tsutsumi, T. Improvement of rutin content in buckwheat flour. In Current Advances in Buckwheat Research, Proceedings of Sixth International Symposium on Buckwheat at Ina, Nagano, Japan, 24–29 August 1995; Matano, T., Ujihara, A., Eds.; Shinshu University Press: Nagano Prefecture, Japan, 1995; pp. 365–372. [Google Scholar]
- Hagels, H. Fagopyrum esculentum Moench. Chemical review. Zbornik BFUL 1999, 73, 29–38. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Wieslander, G.; Norbäck, D. Buckwheat allergy. Allergy 2001, 56, 703–704. [Google Scholar] [CrossRef]




| Country | Tons | Remark | Ranking |
|---|---|---|---|
| Russian Federation | 1524280 | 1 | |
| China | 1447292 | 2 | |
| Ukraine | 180440 | 3 | |
| France | 127406 | IM | 4 |
| Kazakhstan | 120379 | 5 | |
| Poland | 113113 | 6 | |
| United States of America | 76362 | IM | 7 |
| Brazil | 64500 | 8 | |
| Lithuania | 53221 | 9 | |
| Japan | 34400 | 10 | |
| United Republic of Tanzania | 21663 | IM | 11 |
| Belarus | 18010 | 12 | |
| Latvia | 17100 | 13 | |
| Nepal | 12039 | 14 | |
| Bhutan | 3480 | 15 | |
| Estonia | 3385 | 16 | |
| Slovenia | 2909 | 17 | |
| Czechia | 2365 | IM | 18 |
| Republic of Korea | 1633 | IM | 19 |
| Bosnia and Herzegovina | 1187 | 20 | |
| Croatia | 624 | 21 | |
| Hungary | 500 | 22 | |
| Canada | 463 | IM | 23 |
| Slovakia | 367 | 24 | |
| South Africa | 232 | IM | 25 |
| Republic of Moldova | 192 | IM | 26 |
| Georgia | 112 | IM | 27 |
| Kyrgyzstan | 94 | 28 |
| IC50 (μg/mL) * | |
|---|---|
| Crude extract | 20.00 ± 2.67 |
| Rutin | 3.93 ± 0.52 |
| - | Leaves | Flowers |
|---|---|---|
| Rutin | 3417 ± 122 | 822 ± 162 |
| Fagopyrins | 19 ± 2 | 32 ± 4 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtemariam, S. Antioxidant and Rutin Content Analysis of Leaves of the Common Buckwheat (Fagopyrum esculentum Moench) Grown in the United Kingdom: A Case Study. Antioxidants 2019, 8, 160. https://doi.org/10.3390/antiox8060160
Habtemariam S. Antioxidant and Rutin Content Analysis of Leaves of the Common Buckwheat (Fagopyrum esculentum Moench) Grown in the United Kingdom: A Case Study. Antioxidants. 2019; 8(6):160. https://doi.org/10.3390/antiox8060160
Chicago/Turabian StyleHabtemariam, Solomon. 2019. "Antioxidant and Rutin Content Analysis of Leaves of the Common Buckwheat (Fagopyrum esculentum Moench) Grown in the United Kingdom: A Case Study" Antioxidants 8, no. 6: 160. https://doi.org/10.3390/antiox8060160