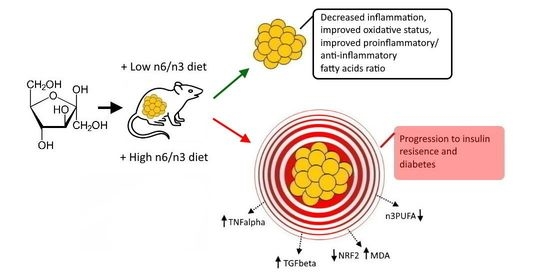
Low Dietary n6/n3 Ratio Attenuates Changes in the NRF 2 Gene Expression, Lipid Peroxidation, and Inflammatory Markers Induced by Fructose Overconsumption in the Rat Abdominal Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Study Design, and Experimental Diets—Experimental Design
2.2. Sample Collection
2.3. Serum Biochemistry
2.4. Determination of Oxidative Status Measurement
2.5. Lipid Extraction and Quantitation of Fatty Acid Composition
2.6. Western Blotting Analysis
2.7. RT-qPCR Analysis
2.8. Statistical Analyses
3. Results
3.1. Body Weight and Insulin Resistance Confirmation
3.2. Biochemical Parameters
3.3. Lipid Peroxidation and NRF 2 Expression
3.4. Expression of Inflammatory Markers and Adiponectin
3.5. Delta 9 Desaturase Expression (Δ9D)
3.6. Adipose Tissue Fatty Acid Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.M.; Jiao, R.Q.; Kong, L.D. High dietary fructose: Direct or indirect dangerous factors disturbing tissue and organ functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Scherer, P.E. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C.; Yaqoob, P. Omega-3 polyunsaturated fatty acids and human health outcomes. BioFactors 2009, 35, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids—A review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef]
- Jump, D.B. Mammalian fatty acid elongases. Methods Mol. Biol. 2009, 579, 375–389. [Google Scholar] [CrossRef] [Green Version]
- Tu, W.C.; Cook-Johnson, R.J.; James, M.J.; Muhlhausler, B.S.; Gibson, R.A. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostag. Leukotr. Essent. 2010, 83, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. 2002, 5, 127–132. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macri, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The anti-inflammatory and antioxidant properties of n-3 pufas: Their role in cardiovascular protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Sakamuri, A.; Sakamuri, S.; Kona, S.R.; Jeyapal, S.; Ibrahim, A. Diets with low n-6:n-3 PUFA ratio protects rats from fructose-induced dyslipidemia and associated hepatic changes: Comparison between 18:3 n-3 and long-chain n-3 PUFA. Prostaglandins Leukot. Essent. Fat. Acids 2020, 155, 102082. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.C. Evaluation of Roche Accu-Chek Go and Medisense Optium blood glucose meters. Clin. Chim. Acta Int. J. Clin. Chem. 2005, 353, 127–131. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Starcevic, K.; Krstulovic, L.; Brozic, D.; Mauric, M.; Stojevic, Z.; Mikulec, Z.; Bajic, M.; Masek, T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015, 95, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Masek, T.; Filipovic, N.; Vuica, A.; Starcevic, K. Effects of treatment with sucrose in drinking water on liver histology, lipogenesis and lipogenic gene expression in rats fed high-fiber diet. Prostag. Leukotr. Essent. 2017, 116, 1–8. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Tapia, E.; Jimenez, A.; Bautista, P.; Cristobal, M.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.J.; et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am. J. Physiol. Ren. Physiol. 2007, 292, F423–F429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, G.C.; Bonner-Weir, S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004, 53, S16–S21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Metabolic phenotyping guidelines: Assessing glucose homeostasis in rodent models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef]
- Karsenty, J.; Landrier, J.F.; Rousseau-Ralliard, D.; Robbez-Masson, V.; Margotat, A.; Deprez, P.; Lechene, P.; Grynberg, A.; Lairon, D.; Planells, R.; et al. Beneficial effects of omega-3 fatty acids on the consequences of a fructose diet are not mediated by PPAR delta or PGC1 alpha. Eur. J. Nutr. 2013, 52, 1865–1874. [Google Scholar] [CrossRef]
- Baena, M.; Sanguesa, G.; Davalos, A.; Latasa, M.J.; Sala-Vila, A.; Sanchez, R.M.; Roglans, N.; Laguna, J.C.; Alegret, M. Fructose, but not glucose, impairs insulin signaling in the three major insulin-sensitive tissues. Sci. Rep. 2016, 6, 26149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starcevic, K.; Roskaric, P.; Speranda, M.; Didara, M.; Kurilj, A.G.; Mauric, M.; Masek, T. High dietary n6/n3 ratio decreases eicosapentaenoic to arachidonic acid ratios and upregulates NFkappaB/p50 expression in short-term low-dose streptozotocin and high-fructose rat model of diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2019, 149, 1–7. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Chiavaroli, L.; Ha, V.; Mirrahimi, A. Fructose vs. glucose and metabolism: Do the metabolic differences matter? Curr. Opin. Lipidol. 2014, 25, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; De Bandt, J.P. Fructose and NAFLD: The multifaceted aspects of fructose metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef] [Green Version]
- Jurgens, H.; Haass, W.; Castaneda, T.R.; Schurmann, A.; Koebnick, C.; Dombrowski, F.; Otto, B.; Nawrocki, A.R.; Scherer, P.E.; Spranger, J.; et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes. Res. 2005, 13, 1146–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa, Y. Adiponectin: Identification, physiology and clinical relevance in metabolic and vascular disease. Atheroscler. Suppl. 2005, 6, 7–14. [Google Scholar] [CrossRef]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Tamura, R.; Kanno, K.; Segawa, T.; Kinoshita, H.; Nishimoto, O.; Nishiyama, H.; Kawamoto, T. Impact of dyslipidemic components of metabolic syndrome, adiponectin levels, and anti-diabetes medications on malondialdehyde-modified low-density lipoprotein levels in statin-treated diabetes patients with coronary artery disease. Diabetol. Metab. Syndr. 2013, 5, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzada, C.; Colas, R.; Guillot, N.; Guichardant, M.; Laville, M.; Véricel, E.; Lagarde, M. Subgram daily supplementation with docosahexaenoic acid protects low-density lipoproteins from oxidation in healthy men. Atherosclerosis 2010, 208, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Guillot, N.; Caillet, E.; Laville, M.; Calzada, C.; Lagarde, M.; Véricel, E. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: Effects on platelet functions and redox status in healthy men. FASEB J. 2009, 23, 2909–2916. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Xiao, B.; Cui, D.; Lin, Y.; Zeng, J.; Li, J.; Cao, M.J.; Liu, J. Antioxidant Activity of Docosahexaenoic Acid (DHA) and Its Regulatory Roles in Mitochondria. J. Agric. Food Chem. 2021, 69, 1647–1655. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef]
- Abrescia, P.; Treppiccione, L.; Rossi, M.; Bergamo, P. Modulatory role of dietary polyunsaturated fatty acids in Nrf2-mediated redox homeostasis. Prog. Lipid Res. 2020, 80, 101066. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.; Yamamoto, K.; Pandey, M.; Loskutoff, D.J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice: A potential role in the obesity-linked increase in plasminogen activator inhibitor-1? Thromb. Haemost. 1997, 3, O1632. [Google Scholar]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharm. 2016, 785, 144–155. [Google Scholar] [CrossRef] [Green Version]
- De Caterina, R.; Cybulsky, M.I.; Clinton, S.K.; Gimbrone, M.A., Jr.; Libby, P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arter. Thromb. 1994, 14, 1829–1836. [Google Scholar] [CrossRef] [Green Version]
- Khalfoun, B.; Thibault, F.; Watier, H.; Bardos, P.; Lebranchu, Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv. Exp. Med. Biol. 1997, 400b, 589–597. [Google Scholar]
- Lo, C.J.; Chiu, K.C.; Fu, M.; Lo, R.; Helton, S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J. Surg. Res. 1999, 82, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Fernandes, G. Decreased pro-inflammatory cytokines and increased antioxidant enzyme gene expression by omega-3 lipids in murine lupus nephritis. Biochem. Biophys. Res. Commun. 1994, 200, 893–898. [Google Scholar] [CrossRef]
- Robinson, D.R.; Urakaze, M.; Huang, R.; Taki, H.; Sugiyama, E.; Knoell, C.T.; Xu, L.; Yeh, E.T.; Auron, P.E. Dietary marine lipids suppress continuous expression of interleukin-1 beta gene transcription. Lipids 1996, 31, S23–S31. [Google Scholar] [CrossRef]
- Turer, A.T.; Scherer, P.E. Adiponectin: Mechanistic insights and clinical implications. Diabetologia 2012, 55, 2319–2326. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kadowaki, T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013, 17, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.; Hodson, L.; Dennis, A.L.; Neville, M.J.; Humphreys, S.M.; Harnden, K.E.; Micklem, K.J.; Frayn, K.N. Markers of de novo lipogenesis in adipose tissue: Associations with small adipocytes and insulin sensitivity in humans. Diabetologia 2009, 52, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Flowers, M.T.; Ntambi, J.M. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr. Opin. Lipidol. 2008, 19, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Flowers, M.T.; Ntambi, J.M. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim. Biophys. Acta 2009, 1791, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Peng, C.; Ai, Y.; Wang, H.; Xiao, X.; Li, J. Docosahexaenoic acid ameliorates fructose-induced hepatic stetosis involving ER stress response in primary mouse hepatocytes. Nutrients 2016, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Hofacer, R.; Magrisso, I.J.; Jandacek, R.; Rider, T.; Tso, P.; Benoit, S.C.; McNamara, R.K. Omega-3 fatty acid deficiency increases stearoyl-CoA desaturase expression and activity indices in rat liver: Positive association with non-fasting plasma triglyceride levels. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, R.R.; Rimoldi, O.J.; Lombardo, Y.B.; Gonzalez, M.S.; Bernasconi, A.M.; Chicco, A.; Basabe, J.C. Desaturase activities in rat model of insulin resistance induced by a sucrose-rich diet. Lipids 2003, 38, 733–742. [Google Scholar] [CrossRef]
- El Hafidi, M.; Cuellar, A.; Ramirez, J.; Banos, G. Effect of sucrose addition to drinking water, that induces hypertension in the rats, on liver microsomal Delta9 and Delta5-desaturase activities. J. Nutr. Biochem. 2001, 12, 396–403. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Nutrients (% Unless Otherwise Stated) | Exp. Diet |
|---|---|
| Crude protein | 20.2 |
| Crude fat | 4.9 |
| Crude fibre | 4.9 |
| Ash | 6.7 |
| Calcium | 1.0 |
| Phosphorus | 0.8 |
| Lysine | 1.2 |
| Methionine | 0.4 |
| ME (MJ)2 | 12.5 |
| Fatty Acids | CON & HF | N6 | DHA |
|---|---|---|---|
| Palmitic (C16:0) | 7.15 | 7.36 | 15.20 |
| Stearic (C18:0) | 2.76 | 2.21 | 4.30 |
| Oleic (C18:1n9) | 28.97 | 29.09 | 31.21 |
| Linoleic (C18:2n6) | 52.25 | 58.69 | 26.78 |
| Linolenic (C18:3n3) | 7.35 | 1.20 | 0.87 |
| Eicosapentaenoic (C20:5n3) | nd | nd | 9.15 |
| Docosahexaenoic (C22:6n3) | nd | nd | 10.98 |
| n6/n3 ratio | 7.11 | 48.91 | 1.28 |
| Gene | Sequence 5′-3′ | Access. No. | Ann. Tm. | Cycles |
|---|---|---|---|---|
| TGF β | F: AAT ACG TCA GAC ATT CGG GAA GCA | NM_021578.2 | 60 °C | 40 |
| R: AAT ACG TCA GAC ATT CGG GAA GCA | ||||
| TNF α | F: CAC CAC GCT CTT CTG TCT ACT GAA C | NM_012675.3 | 60 °C | 40 |
| R: CCG GAC TCC GTG ATG TCT AAG TAC T | ||||
| Δ9D | F: ACA TTC AAT CTC GGG AGA ACA | NM_139192.2 | 60 °C | 40 |
| R: CCA TGC AGT CGA TGA AGA AC | ||||
| NRF 2 | F: CAC ATC CAG ACA GAC ACC AGT | NM_031789.2 | 60 °C | 40 |
| R: CTA CAA ATG GGA ATG TCT CTG C | ||||
| β-actin | F: CAT TGT CAC CAA CTG GGA CGA TA | XM_039089807.1 | 60 °C | 40 |
| R: GGA TGG CTA CGT ACA TGG CTG | ||||
| Cyclophilin | F: GGA TGG CAA GCA TGT GGT CTT TG | M19533 | 60 °C | 40 |
| R: CTT CTT GCT GGT CTT GCC ATT CCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roškarić, P.; Šperanda, M.; Mašek, T.; Verbanac, D.; Starčević, K. Low Dietary n6/n3 Ratio Attenuates Changes in the NRF 2 Gene Expression, Lipid Peroxidation, and Inflammatory Markers Induced by Fructose Overconsumption in the Rat Abdominal Adipose Tissue. Antioxidants 2021, 10, 2005. https://doi.org/10.3390/antiox10122005
Roškarić P, Šperanda M, Mašek T, Verbanac D, Starčević K. Low Dietary n6/n3 Ratio Attenuates Changes in the NRF 2 Gene Expression, Lipid Peroxidation, and Inflammatory Markers Induced by Fructose Overconsumption in the Rat Abdominal Adipose Tissue. Antioxidants. 2021; 10(12):2005. https://doi.org/10.3390/antiox10122005
Chicago/Turabian StyleRoškarić, Petra, Marcela Šperanda, Tomislav Mašek, Donatella Verbanac, and Kristina Starčević. 2021. "Low Dietary n6/n3 Ratio Attenuates Changes in the NRF 2 Gene Expression, Lipid Peroxidation, and Inflammatory Markers Induced by Fructose Overconsumption in the Rat Abdominal Adipose Tissue" Antioxidants 10, no. 12: 2005. https://doi.org/10.3390/antiox10122005